
Topicity
Encyclopedia
In chemistry, topicity is the stereochemical relationship of substituents relative to the structure to which they are attached. Depending on the relationship, such groups can be heterotopic, homotopic, enantiotopic, or diastereotopic.
spectrum. For example, the four hydrogen atoms of methane
(CH4) are homotopic with one another, as are the two hydrogens or the two chlorines in dichloromethane
(CH2Cl2).
term enantiotopic refers to the relationship between two groups in a molecule which, if one or the other were replaced, would generate a chiral
compound. The two possible compounds resulting from that replacement would be enantiomer
s.
For example, the two hydrogen atoms attached to the second carbon in butane
are enantiotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (R)-2-bromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the enantiomer (S)-2-bromobutane.
Enantiotopic groups are identical and indistinguishable except in chiral environments. For instance, the CH2 hydrogens in ethanol
(CH3CH2OH) are normally enantiotopic, but can be made different (diastereotopic) if combined with a chiral center, for instance by conversion to an ester
of a chiral carboxylic acid
such as lactic acid
, or if coordinated to a chiral metal center, or if associated with an enzyme
active site
, since enzymes are constituted of chiral amino acid
s. Indeed, in the presence of the enzyme LADH, one specific hydrogen is removed from the CH2 group during the oxidation of ethanol to acetaldehyde
, and it gets replaced in the same place during the reverse reaction. The chiral environment needs not be optically pure for this effect.
Enantiotopic groups are mirror images of each other about an internal plane of symmetry. A chiral environment removes that symmetry. Enantiotopic pairs of NMR-active nuclei are also indistinguishable by NMR and produce a single signal.
Enantiotopic groups need not be attached to the same atom. For example, two hydrogen atoms adjacent to the carbonyl group in cis-2,6-dimethylcyclohexanone are enantiotopic; they are related by an internal plane of symmetry passing through the carbonyl group, but deprotonation on one side of the carbonyl group or on the other will generate compounds which are enantiomers. Similarly, replacement of one or the other with deuterium
will generate enantiomers.
term diastereotopic refers to the relationship between two groups in a molecule which, if replaced, would generate compounds that are diastereomer
s. Diastereotopic groups are often, but not always, identical groups attached to the same atom in a molecule containing at least one chiral center.
For example, the two hydrogen atoms of the CH2 moiety in (S)-2-bromobutane are diastereotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (2S,3R)-2,3-dibromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the diastereomer (2S,3S)-2,3-dibromobutane.
In chiral molecules containing diastereotopic groups, such as in 2-bromobutane, there is no requirement for enantiomeric or optical purity; no matter its proportion, each enantiomer will generate enantiomeric sets of diastereomers upon substitution of diastereotopic groups (though, as in the case of substitution by bromine in 2-bromobutane, meso
isomers have, strictly speaking, no enantiomer).
Diastereotopic groups are not mirror images of one another about any plane. They are always different, in any environment, but may not be distinguishable. For instance, both pairs of CH2 hydrogens in ethyl phenylalaninate hydrochloride (PhCH2CH(NH3+)COOCH2CH3 Cl-) are diastereotopic and both give pairs of distinct 1H-NMR signals in DMSO-d6 at 300 MHz, but in the similar ethyl 2-nitrobutanoate (CH3CH2CH(NO2)COOCH2CH3), only the CH2 group next to the chiral center gives distinct signals from its two hydrogens with the same instrument in CDCl3. Such signals are often complex because of small differences in chemical shift, overlap and an additional strong coupling between geminal
hydrogens. On the other hand, the two CH3 groups of ipsenol, which are three bonds away from the chiral center, give separate 1H doublets at 300 MHz and separate 13C-NMR signals in CDCl3, but the diastereotopic hydrogens in ethyl alaninate hydrochloride (CH3CH(NH3+)COOCH2CH3 Cl-), also three bonds away from the chiral center, show barely distinguishable 1H-NMR signals in DMSO-d6.
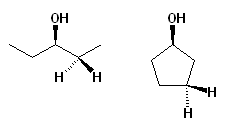 Diastereotopic groups also arise in achiral molecules. For instance, any one pair of CH2 hydrogens in 3-pentanol (Figure 1) are diastereotopic, as the two CH2 carbons are enantiotopic. Substitution of any one of the four CH2 hydrogens creates two chiral centers at once, and the two possible hydrogen substitution products at any one CH2 carbon will be diastereomers. This kind of relationship is often easier to detect in cyclic molecules. For instance, any pair of CH2 hydrogens in cyclopentanol (Figure 1) are similarly diastereotopic, and this is easily discerned as one of the hydrogens in the pair will be cis to the OH group (on the same side of the ring face) while the other will be trans to it (on the opposite side).
Diastereotopic groups also arise in achiral molecules. For instance, any one pair of CH2 hydrogens in 3-pentanol (Figure 1) are diastereotopic, as the two CH2 carbons are enantiotopic. Substitution of any one of the four CH2 hydrogens creates two chiral centers at once, and the two possible hydrogen substitution products at any one CH2 carbon will be diastereomers. This kind of relationship is often easier to detect in cyclic molecules. For instance, any pair of CH2 hydrogens in cyclopentanol (Figure 1) are similarly diastereotopic, and this is easily discerned as one of the hydrogens in the pair will be cis to the OH group (on the same side of the ring face) while the other will be trans to it (on the opposite side).
The term diastereotopic is also applied to identical groups attached to the same end of an alkene moiety which, if replaced, would generate geometric isomers (also falling in the category of diastereomers). Thus, the CH2 hydrogens of propene are diastereotopic, one being cis to the CH3 group, and the other being trans to it, and replacement of one or the other with CH3 would generate cis- or trans--2-butene.
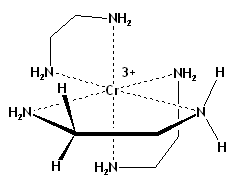 Diastereotopicity is not limited to organic molecules, nor to groups attached to carbon, nor to molecules with chiral tetrahedral (sp3-hybridized) centers: for instance, the pair of hydrogens in any CH2 or NH2 group in tris(ethylenediamine)chromium(III) ion (Cr(en)33+), where the metal center is chiral, are diastereotopic (Figure 2).
Diastereotopicity is not limited to organic molecules, nor to groups attached to carbon, nor to molecules with chiral tetrahedral (sp3-hybridized) centers: for instance, the pair of hydrogens in any CH2 or NH2 group in tris(ethylenediamine)chromium(III) ion (Cr(en)33+), where the metal center is chiral, are diastereotopic (Figure 2).
The terms enantiotopic and diastereotopic can also be applied to the faces of planar groups (especially carbonyl groups and alkene moities). See Cahn-Ingold-Prelog priority rule
.
Homotopic
Homotopic groups in a chemical compound are equivalent groups. Two groups A and B are homotopic if the molecule remains the same (including stereochemically) when the groups are interchanged with the remaining parts of the molecule fixed. Homotopic atoms are always identical, in any environment. Homotopic NMR-active nuclei have the same chemical shift in an NMRNuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
spectrum. For example, the four hydrogen atoms of methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
(CH4) are homotopic with one another, as are the two hydrogens or the two chlorines in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
(CH2Cl2).
Enantiotopic
The stereochemicalStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
term enantiotopic refers to the relationship between two groups in a molecule which, if one or the other were replaced, would generate a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
compound. The two possible compounds resulting from that replacement would be enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s.
For example, the two hydrogen atoms attached to the second carbon in butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
are enantiotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (R)-2-bromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the enantiomer (S)-2-bromobutane.
 |
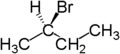 |
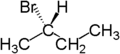 |
| Butane | (R)-2-bromobutane | (S)-2-bromobutane |
Enantiotopic groups are identical and indistinguishable except in chiral environments. For instance, the CH2 hydrogens in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
(CH3CH2OH) are normally enantiotopic, but can be made different (diastereotopic) if combined with a chiral center, for instance by conversion to an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
of a chiral carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
such as lactic acid
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
, or if coordinated to a chiral metal center, or if associated with an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
, since enzymes are constituted of chiral amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. Indeed, in the presence of the enzyme LADH, one specific hydrogen is removed from the CH2 group during the oxidation of ethanol to acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
, and it gets replaced in the same place during the reverse reaction. The chiral environment needs not be optically pure for this effect.
Enantiotopic groups are mirror images of each other about an internal plane of symmetry. A chiral environment removes that symmetry. Enantiotopic pairs of NMR-active nuclei are also indistinguishable by NMR and produce a single signal.
Enantiotopic groups need not be attached to the same atom. For example, two hydrogen atoms adjacent to the carbonyl group in cis-2,6-dimethylcyclohexanone are enantiotopic; they are related by an internal plane of symmetry passing through the carbonyl group, but deprotonation on one side of the carbonyl group or on the other will generate compounds which are enantiomers. Similarly, replacement of one or the other with deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
will generate enantiomers.
Diastereotopic
The stereochemicalStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
term diastereotopic refers to the relationship between two groups in a molecule which, if replaced, would generate compounds that are diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s. Diastereotopic groups are often, but not always, identical groups attached to the same atom in a molecule containing at least one chiral center.
For example, the two hydrogen atoms of the CH2 moiety in (S)-2-bromobutane are diastereotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (2S,3R)-2,3-dibromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the diastereomer (2S,3S)-2,3-dibromobutane.
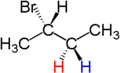 |
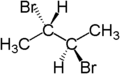 |
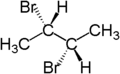 |
| (S)-2-bromobutane | (2S,3R)-2,3-dibromobutane | (2S,3S)-2,3-dibromobutane |
In chiral molecules containing diastereotopic groups, such as in 2-bromobutane, there is no requirement for enantiomeric or optical purity; no matter its proportion, each enantiomer will generate enantiomeric sets of diastereomers upon substitution of diastereotopic groups (though, as in the case of substitution by bromine in 2-bromobutane, meso
Meso
Meso may refer to:*meso-, a prefix meaning middle or intermediate*Meso compound, a stereochemical classification in chemistry*Meso, the currency in the 2d online game MapleStory*Mesopotamia, the first major river civilization, known today as Iraq....
isomers have, strictly speaking, no enantiomer).
Diastereotopic groups are not mirror images of one another about any plane. They are always different, in any environment, but may not be distinguishable. For instance, both pairs of CH2 hydrogens in ethyl phenylalaninate hydrochloride (PhCH2CH(NH3+)COOCH2CH3 Cl-) are diastereotopic and both give pairs of distinct 1H-NMR signals in DMSO-d6 at 300 MHz, but in the similar ethyl 2-nitrobutanoate (CH3CH2CH(NO2)COOCH2CH3), only the CH2 group next to the chiral center gives distinct signals from its two hydrogens with the same instrument in CDCl3. Such signals are often complex because of small differences in chemical shift, overlap and an additional strong coupling between geminal
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
hydrogens. On the other hand, the two CH3 groups of ipsenol, which are three bonds away from the chiral center, give separate 1H doublets at 300 MHz and separate 13C-NMR signals in CDCl3, but the diastereotopic hydrogens in ethyl alaninate hydrochloride (CH3CH(NH3+)COOCH2CH3 Cl-), also three bonds away from the chiral center, show barely distinguishable 1H-NMR signals in DMSO-d6.

The term diastereotopic is also applied to identical groups attached to the same end of an alkene moiety which, if replaced, would generate geometric isomers (also falling in the category of diastereomers). Thus, the CH2 hydrogens of propene are diastereotopic, one being cis to the CH3 group, and the other being trans to it, and replacement of one or the other with CH3 would generate cis- or trans--2-butene.

The terms enantiotopic and diastereotopic can also be applied to the faces of planar groups (especially carbonyl groups and alkene moities). See Cahn-Ingold-Prelog priority rule
Cahn-Ingold-Prelog priority rule
The Cahn–Ingold–Prelog priority rules, CIP system or CIP conventions are a set of rules used in organic chemistry to name the stereoisomers of a molecule. A molecule may contain any number of stereocenters and any number of double bonds, and each gives rise to two possible configurations...
.

