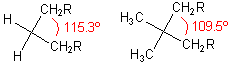
Thorpe-Ingold effect
Encyclopedia
The Thorpe–Ingold effect or gem-dimethyl effect, or angle compression is an effect observed in organic chemistry
where increasing the size of two substituents on a tetrahedral center leads to enhanced reactions between parts of the other two substituents. The effect was first reported by Beesley, Thorpe and Ingold in 1916 as part of a study of cyclization reactions.
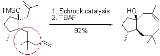
A common application of this effect is addition of a quaternary carbon (e.g., a gem
-dimethyl group
) in an alkyl chain to increase the reaction rate
and/or equilibrium constant of cyclization reactions. An example of this is an olefin metathesis
reaction:
One proposed explanation for this effect is that the increased size of the substituents increases the angle between them. As a result, the angle between the other two substituents decreases. By moving them closer together, reactions between them are accelerated. It is thus a kinetic effect.
The effect also has some thermodynamic contribution as the in silico
strain energy
decreases on going from cyclobutane
to 1-methylcyclobutane and 1,1-dimethylcyclobutane by a value between 8 kcal/mole and 1.5 kcal/mole.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
where increasing the size of two substituents on a tetrahedral center leads to enhanced reactions between parts of the other two substituents. The effect was first reported by Beesley, Thorpe and Ingold in 1916 as part of a study of cyclization reactions.
A common application of this effect is addition of a quaternary carbon (e.g., a gem
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
-dimethyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
) in an alkyl chain to increase the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
and/or equilibrium constant of cyclization reactions. An example of this is an olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
reaction:
One proposed explanation for this effect is that the increased size of the substituents increases the angle between them. As a result, the angle between the other two substituents decreases. By moving them closer together, reactions between them are accelerated. It is thus a kinetic effect.
The effect also has some thermodynamic contribution as the in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
decreases on going from cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
to 1-methylcyclobutane and 1,1-dimethylcyclobutane by a value between 8 kcal/mole and 1.5 kcal/mole.