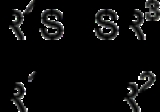
Thioketal
Encyclopedia
In chemistry, a thioketal is the sulfur
analogue of a ketal, with one of the oxygen
replaced by sulfur. A dithioketal has both oxygens replaced by sulfur.
Thioketals can be obtained by reacting ketones or aldehydes with thioles.
Ketones can be reduced at neutral pH using the thioketal method. The thioketal prepared from the ketone can be easily reduced by catalytic hydrogenation.
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
analogue of a ketal, with one of the oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
replaced by sulfur. A dithioketal has both oxygens replaced by sulfur.
Thioketals can be obtained by reacting ketones or aldehydes with thioles.
Ketones can be reduced at neutral pH using the thioketal method. The thioketal prepared from the ketone can be easily reduced by catalytic hydrogenation.

