
Surface extended X-ray absorption fine structure
Encyclopedia
Surface extended X-ray absorption fine structure is the surface sensitive equivalent of the EXAFS technique. This technique involves the illumination of the sample by high intensity X-ray
beams from a synchrotron
and monitoring their photoabsorption by detecting in the intensity of Auger electron
s as a function of the incident photon energy. Surface sensitivity is achieved by the fact that the interpretation of data depends on the intensity of the Auger electrons (which have an escape depth of ~1–2 nm
) instead of looking at the relative absorption of the X-rays as in the parent method, EXAFS.
The photon energies are tuned through the characteristic energy for the onset of core level
excitation for surface atoms. The core holes thus created can then be filled by nonradiative decay of a higher lying electron and communication of energy to yet another electron, which can then escape from the surface (Auger emission). The photoabsorption can therefore be monitored by direct detection of these Auger electrons to the total photoelectron yield. The absorption coefficient versus incident photon energy contains oscillations which are due to the interference of the backscattered Auger electrons with the outward propagating waves. The period of this oscillations depends on the type of the backscattering atom and its distance from the central atom. Thus, this technique enables the investigation of interatomic distances for adsorbates and their coordination chemistry.
This technique benefits from the fact that long range order is not required which sometimes becomes a limitation in the other conventional techniques like LEED (about 10 nm). This method also largely eliminates the background from the signal. It also benefits from the fact that it can probe different species in the sample by just tuning the X-ray photon energy to the absorption edge of that species. Joachim Stöhr
played a major role in the initial development of this technique.
radiation as it has highly collimated, plane polarized and precisely pulsed X-ray sources, with fluxes of 1012 to 1014 photons/sec/mrad/mA and greatly improves the signal-to-noise ratio over than obtainable from conventional sources. The experimental setup for the conventional EXAFS is shown here in Figure 2. A bright source X-ray source is illuminating the sample and the transmission is being measured as the absorption coefficient as
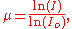
where I is the transmitted and Io is the incident intensity of the X-rays. Then it is plotted against the energy of the incoming X-ray photon energy.
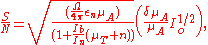
where
This excitation also triggers various decay mechanisms. These can be of radiative (fluorescence) or nonradiative (Auger and Coster–Kronig) nature. The intensity ratio between the Auger electron and x-ray emissions depends on the atomic number Z. The yield of the Auger electrons decreases with increasing Z.

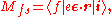
where the initial state, i with energy Ei, consists of the atomic core and the Fermi sea, and the incident radiation field, the final state, ƒ with energy Eƒ (larger than the Fermi level), consists of a core hole and an excited electron. ε is the polarization vector of the electric field, e the electron charge, and ħω the x-ray photon energy. The photoabsorption signal contains a peak when the core level excitation is neared. It is followed by an oscillatory component which originates from the coupling of that part of the electron wave which upon scattering by the medium is turned back towards the central ionized atom, where it couples to the initial state via the dipole operator, Mi.
Assuming single-scattering and small atom approximation for kRj >> 1, where Rj is the distance from the central excited atom to the jth shell of neighbors and k is the photoelectrons wave vector,

where ħωT is the absorption edge energy and Vo is the inner potential of the solid associated with exchange and correlation, the following expression for the oscillatory component of the photoabsorption cross section (for K-shell excitation) is obtained:
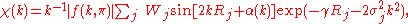
where the atomic scattering factor in a partial wave expansion with partial wave phase-shifts δl is given by
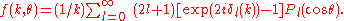
Pl(x) is the lth Legendre polynomial, γ is an attenuation coefficient, exp(−2σi2k2) is a Debye-Waller factor
and weight Wj is given in terms of the number of atoms in the jth shell and their distance as
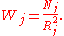
The above equation for the χ(k) forms the basis of a direct, Fourier transform, method of analysis which has been successfully applied to the analysis of the EXAFS data.
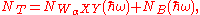
where NB(ħω) is the background signal and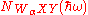 is the Auger signal we are interested in, where
is the Auger signal we are interested in, where
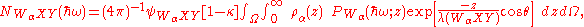
where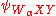 is the probability that an excited atom will decay via WαXY Auger transition, ρα(z) is the atomic concentration of the element α at depth z, λ(WαXY) is the mean free path for an WαXY Auger electron, θ is the angle that the escaping Auger electron makes with the surface normal and κ is the photon emission probability which is dictated the atomic number. As the photoabsorption probability,
is the probability that an excited atom will decay via WαXY Auger transition, ρα(z) is the atomic concentration of the element α at depth z, λ(WαXY) is the mean free path for an WαXY Auger electron, θ is the angle that the escaping Auger electron makes with the surface normal and κ is the photon emission probability which is dictated the atomic number. As the photoabsorption probability,  is the only term that is dependent on the photon energy, the oscillations in it as a function of eneryg would give rise to similar oscillations in the
is the only term that is dependent on the photon energy, the oscillations in it as a function of eneryg would give rise to similar oscillations in the 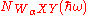 .
.
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
beams from a synchrotron
Synchrotron
A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant...
and monitoring their photoabsorption by detecting in the intensity of Auger electron
Auger electron
The Auger effect is a physical phenomenon in which the transition of an electron in an atom filling in an inner-shell vacancy causes the emission of another electron. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in...
s as a function of the incident photon energy. Surface sensitivity is achieved by the fact that the interpretation of data depends on the intensity of the Auger electrons (which have an escape depth of ~1–2 nm
Nanometre
A nanometre is a unit of length in the metric system, equal to one billionth of a metre. The name combines the SI prefix nano- with the parent unit name metre .The nanometre is often used to express dimensions on the atomic scale: the diameter...
) instead of looking at the relative absorption of the X-rays as in the parent method, EXAFS.
The photon energies are tuned through the characteristic energy for the onset of core level
Core electron
Core electrons are the electrons in an atom that are not valence electrons and therefore do not participate in bonding. An example: the carbon atom has a total of 6 electrons, 4 of them being valence electrons. So the remaining 2 electrons must be core electrons.They are so tightly bound to the...
excitation for surface atoms. The core holes thus created can then be filled by nonradiative decay of a higher lying electron and communication of energy to yet another electron, which can then escape from the surface (Auger emission). The photoabsorption can therefore be monitored by direct detection of these Auger electrons to the total photoelectron yield. The absorption coefficient versus incident photon energy contains oscillations which are due to the interference of the backscattered Auger electrons with the outward propagating waves. The period of this oscillations depends on the type of the backscattering atom and its distance from the central atom. Thus, this technique enables the investigation of interatomic distances for adsorbates and their coordination chemistry.
This technique benefits from the fact that long range order is not required which sometimes becomes a limitation in the other conventional techniques like LEED (about 10 nm). This method also largely eliminates the background from the signal. It also benefits from the fact that it can probe different species in the sample by just tuning the X-ray photon energy to the absorption edge of that species. Joachim Stöhr
Joachim Stöhr
Joachim Stöhr received his Ph.D. in Physics from the Technical University of Munich. After postdoctoral work at Lawrence Berkeley National Laboratory he worked as a staff scientist at the Stanford Synchrotron Radiation Laboratory and at EXXON Corporate Research Laboratory...
played a major role in the initial development of this technique.
Synchrotron radiation sources
Normally, the SEXAFS work is done using synchrotronSynchrotron
A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant...
radiation as it has highly collimated, plane polarized and precisely pulsed X-ray sources, with fluxes of 1012 to 1014 photons/sec/mrad/mA and greatly improves the signal-to-noise ratio over than obtainable from conventional sources. The experimental setup for the conventional EXAFS is shown here in Figure 2. A bright source X-ray source is illuminating the sample and the transmission is being measured as the absorption coefficient as
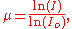
where I is the transmitted and Io is the incident intensity of the X-rays. Then it is plotted against the energy of the incoming X-ray photon energy.
Electron detectors
In SEXAFS, an electron detector and a high vacuum chamber is required to calculate the Auger yields instead of the intensity of the transmitted X-ray waves. The detector can be either an energy analyzer, as in the case of Auger measurements, or an electron multiplier, as in the case of total or partial secondary electron yield. The energy analyzer gives rise to better resolution while the electron multiplier has larger solid angle acceptance.Signal to noise ratio
The equation governing the signal to noise ratio is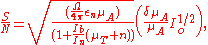
where
- μA is the absorption coefficient;
- In is the nonradiative contribution in electron counts/sec;
- Ib is the background contribution in electron counts/sec;
- μA is the absorption by the SEXAFS-producing element;
- μT is the total absorption by all the elements;
- Io is the incident intensity;
- n is the attenuation length;
- Ω/(4π) is the solid angle acceptance for the detector;
- εn is the nonradiative yield which is the probability that the electron will not decay radiatively and will actually get emitted as an Auger electron.
Basics
The absorption of a X-ray photon by the atom excites a core level electron thus generating a core hole. This generates a spherical electron wave with the excited atom as the center. This wave propagates outwards and get scattered off from the neighbouring atoms and is turned back towards the central ionized atom. The oscillatory component of the photoabsorption originates from the coupling of this reflected wave to the initial state via the dipole operator Mfs as in (1). The Fourier transform of the oscillations gives us the information about the spacing of the neighboring atoms and their chemical environment. This phase information is carried over to the oscillations in the Auger signal because the transition time in Auger emission is of the same order of magnitude as the average time for a photoelectron in the energy range of interest. Thus, with a proper choice of the absorption edge and characteristic Auger transition, measurement of the variation of the intensity in a particular Auger line as a function of incident photon energy would be a measure of the photoabsorption cross section.This excitation also triggers various decay mechanisms. These can be of radiative (fluorescence) or nonradiative (Auger and Coster–Kronig) nature. The intensity ratio between the Auger electron and x-ray emissions depends on the atomic number Z. The yield of the Auger electrons decreases with increasing Z.
Theory of EXAFS
The cross section of photoabsorption is given by the Fermi golden rule formula, which, in the dipole approximation, is given as
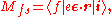
where the initial state, i with energy Ei, consists of the atomic core and the Fermi sea, and the incident radiation field, the final state, ƒ with energy Eƒ (larger than the Fermi level), consists of a core hole and an excited electron. ε is the polarization vector of the electric field, e the electron charge, and ħω the x-ray photon energy. The photoabsorption signal contains a peak when the core level excitation is neared. It is followed by an oscillatory component which originates from the coupling of that part of the electron wave which upon scattering by the medium is turned back towards the central ionized atom, where it couples to the initial state via the dipole operator, Mi.
Assuming single-scattering and small atom approximation for kRj >> 1, where Rj is the distance from the central excited atom to the jth shell of neighbors and k is the photoelectrons wave vector,

where ħωT is the absorption edge energy and Vo is the inner potential of the solid associated with exchange and correlation, the following expression for the oscillatory component of the photoabsorption cross section (for K-shell excitation) is obtained:
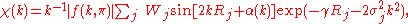
where the atomic scattering factor in a partial wave expansion with partial wave phase-shifts δl is given by
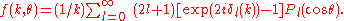
Pl(x) is the lth Legendre polynomial, γ is an attenuation coefficient, exp(−2σi2k2) is a Debye-Waller factor
Debye-Waller factor
The Debye–Waller factor , named after Peter Debye and Ivar Waller, is used in condensed matter physics to describe the attenuation of x-ray scattering or coherent neutron scattering caused by thermal motion. It has also been called the B factor or the temperature factor...
and weight Wj is given in terms of the number of atoms in the jth shell and their distance as
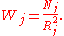
The above equation for the χ(k) forms the basis of a direct, Fourier transform, method of analysis which has been successfully applied to the analysis of the EXAFS data.
Incorporation of EXAFS-Auger
The number of electrons arriving at the detector with an energy of the characteristic WαXY Auger line (where Wα is the absorption edge core-level of element α, to which the incident x-ray line has been tuned) can be written as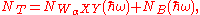
where NB(ħω) is the background signal and
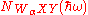 is the Auger signal we are interested in, where
is the Auger signal we are interested in, where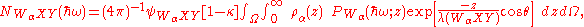
where
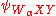 is the probability that an excited atom will decay via WαXY Auger transition, ρα(z) is the atomic concentration of the element α at depth z, λ(WαXY) is the mean free path for an WαXY Auger electron, θ is the angle that the escaping Auger electron makes with the surface normal and κ is the photon emission probability which is dictated the atomic number. As the photoabsorption probability,
is the probability that an excited atom will decay via WαXY Auger transition, ρα(z) is the atomic concentration of the element α at depth z, λ(WαXY) is the mean free path for an WαXY Auger electron, θ is the angle that the escaping Auger electron makes with the surface normal and κ is the photon emission probability which is dictated the atomic number. As the photoabsorption probability,  is the only term that is dependent on the photon energy, the oscillations in it as a function of eneryg would give rise to similar oscillations in the
is the only term that is dependent on the photon energy, the oscillations in it as a function of eneryg would give rise to similar oscillations in the 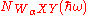 .
.

