
Sulfinic acids
Encyclopedia
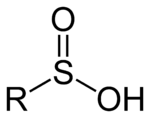
Oxoacid
An oxoacid is an acid that contains oxygen. To be more specific, it is an acid that:#contains oxygen#contains at least one other element#has at least one hydrogen atom bound to oxygen#forms an ion by the loss of one or more protons....
s of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal, thus the acids are chiral.
Because these acids are often fragile, they are often prepared in situ by acidification of the corresponding sulfinate salts, which are robust. These salts are generate by reduction of sulfonyl chlorides. Alternative routes include treating Grignard reagents with sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
. Transition metal sulfinates are also generated by insertion of sulfur dioxide into metal alkyls, a reaction that proceeds via a metal sulfur dioxide complex
Metal sulfur dioxide complex
Metal sulfur dioxide complexes are complexes that contain sulfur dioxide, SO2, bonded to a transition metal. Such compounds are common but are mainly of theoretical interest...
.
Examples
An example of a well-studied sulfinic acid is phenylsulfinic acidPhenylsulfinic acid
Phenylsulfinic acid is an organosulfur compound with the formula C6H5SO2H. It is a colorless, or white crystalline solid that is usually stored in the form of its sodium salt. In aqueous solution it is strongly acidic and is easily oxidized in air...
. A commercially important sulfinic acid is thiourea dioxide
Thiourea dioxide
Thiourea dioxide is an organosulfur compound that is used in the textile industry. It functions as a reducing agent. Thiourea dioxide is not a dioxide, but instead is a derivative of a sulfinic acid Thiourea dioxide is an organosulfur compound that is used in the textile industry. It functions as...
.

