Sulfamide
Encyclopedia
Sulfamide is a chemical compound with the molecular structure H2NSO2NH2. Sulfamide is produced by the reaction of sulfuryl chloride
with ammonia
.
, the term sulfamide may also refer to the functional group
which consists of at least one organic group attached to a nitrogen atom of sulfamide.
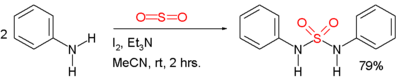
Symmetric sulfamides can be prepared directly from amine
s and sulfur dioxide
gas:
In this example, the reactants are aniline
, triethylamine
, and iodine
. Sulfur dioxide is believed to be activated through a series of intermediates: Et3N-I+-I-, Et3N-I+-I3- and Et3N+-SO2-.
The sulfamide functional group is an increasingly common structural feature used in medicinal chemistry.
Sulfuryl chloride
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis....
with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
.
Sulfamide functional group.
In organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, the term sulfamide may also refer to the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
which consists of at least one organic group attached to a nitrogen atom of sulfamide.
Symmetric sulfamides can be prepared directly from amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s and sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
gas:
In this example, the reactants are aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
, triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
, and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
. Sulfur dioxide is believed to be activated through a series of intermediates: Et3N-I+-I-, Et3N-I+-I3- and Et3N+-SO2-.
The sulfamide functional group is an increasingly common structural feature used in medicinal chemistry.