
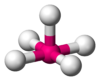
Square pyramidal molecular geometry
Encyclopedia
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
, square based pyramidal geometry describes the shape of certain compounds with the formula ML5 where L is a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The geometry is common for certain main group compounds that have a stereochemically active lone pair, as described by VSEPR theory
VSEPR theory
Valence shell electron pair repulsion theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion. It is also named Gillespie–Nyholm theory after its two main developers...
. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably [Ni(CN)5]3−.
As a transition state in Berry Pseudorotation
As a trigonal bipyramidal molecule undergoes Berry pseudorotation, it proceeds via an intermediary stage with the square planar geometry. Thus even though the geometry is rarely seen as the ground state, it is accessed by a low energy distortion from a trigonal bipyramid.Pseudorotation also occurs in square pyramidal molecules. Molecules with this geometry, as opposed to trigonal bipyramidal, exhibit heavier vibration. The mechanism used is similar to the Berry mechanism.
Examples
Some molecular compounds that adopt square pyramidal geometry are XeOF4, and XF5 (X = Cl, Br, I) also have square pyramidal geometries. Complexes of vanadiumVanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
(IV), such as [VO(acac)2] are square pyramidal (acac = acetylacetonate, the anion of 2,4-pentanedione that has lost a proton).

