
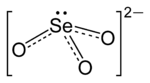
Selenite (ion)
Encyclopedia
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
oxoanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
SeO32−.
A selenite (compound) is a compound that contains this ion.
In slightly acid conditions, the hydrogenselenite ion, HSeO3−, is formed; in more acidic conditions selenous acid
Selenous acid
Selenous acid is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by 2SeO. It is the principal oxoacid of selenium; the other being selenic acid.-Formation and properties:...
, H2SeO3, exists.
Most selenite salts can be formed by heating the relevant metal oxide with selenium dioxide
Selenium dioxide
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.-Properties:...
, e.g.:
- Na2O + SeO2 → Na2SeO3.
See :Category:Selenites for a list.

