
Saturated absorption
Encyclopedia
In experimental physics, saturated absorption is a set-up that enables the precise determination of the transition frequency of an atom
between its ground state and an optically excited state. The accuracy to which these frequencies can be determined should ideally be only limited by the width of the excited state
, which is the inverse of the lifetime of this state. However, the samples of atomic gas that are used to that purpose are at room temperature, where the measured frequency distribution is highly broadened due to the Doppler effect
. Saturated absorption allows precise spectroscopy of the atomic levels without having to cool the sample down to temperatures where the Doppler broadening is no longer relevant (which would be on the order of a few millikelvins). It is also used to lock the frequency of a laser
to the precise wavelength of an atom in atomic physics experiments.
, the atom absorption of light depends on the frequency of the incident field. More precisely, the absorption is characterized by a lorentzian
of width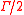 (
( MHz for the atom of Rubidium
MHz for the atom of Rubidium
for example). If we have a cell of atomic vapour at room temperature, then the distribution of velocity will follow a Maxwell–Boltzmann distribution
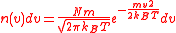
where N is the number of atoms,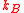 the Boltzmann constant, m the mass of the atom. According to the Doppler effect
the Boltzmann constant, m the mass of the atom. According to the Doppler effect
formula in the case of non-relativistic speeds
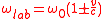
where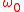 is the frequency of the atom at rest (the one which is being probed). The value of v as a function of
is the frequency of the atom at rest (the one which is being probed). The value of v as a function of 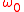 and
and 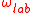 can be inserted in the distribution of velocities. The distribution of absorption as a function of the pulsation will therefore be proportional to a Gaussian of full width at half maximum
can be inserted in the distribution of velocities. The distribution of absorption as a function of the pulsation will therefore be proportional to a Gaussian of full width at half maximum
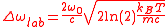
For a Rubidium atom at room temperature,
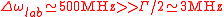
Therefore without any special trick in the experimental setup probing the maximum of absorption of an atomic vapour, the uncertainty of the measurement will be limited by the Doppler broadening and not by the fundamental width of the resonance.
If the frequency is slightly red-detuned
with respect to the atomic frequency, the pump beam will be absorbed by the atoms moving towards its source, the +x direction, while the probe beam will be absorbed by the atoms moving in the -x direction (counter-propagating beams and Doppler effect) which are different from those that interact with the pump beam. On the contrary, if the frequency of the lasers is slightly blue-detuned, the pump beam will be absorbed by the atoms moving away from it in the -x direction, and the probe beam by other atoms moving in the +x direction.
Now let us consider the case of a frequency exactly tuned at the atom frequency. The atoms whose velocity along the x direction is zero will then interact with both the pump and probe beams, which are both resonant with them. Due to its important power, the pump beam will saturate the atomic transition. We can thus consider that in a significant number of cases, a photon coming from the probe beam and arriving on an atom will find it in its excited state. Due to the process of stimulated emission
, this photon will desexcite the atom and go through the sample. Thus the probe beam is not completely absorbed at the atomic frequency. On the photodiode, one would then expect to see a Gaussian profile (due to Doppler broadening) with a small dimple in the middle due to the stimulated emission phenomena. This dimple indicates precisely the frequency of the atomic transition.
. The probe beam can be made of the light reflected by a glass slab (so that its power is much weaker than the power of the pump beam). Some additional modulation can be added either to the laser or to the atoms (thanks for example to a magnetic field) to ease the signal processing afterwards (especially when using a synchronous detection).
The description of the principle of saturated absorption used only a two-level atom. However in real atoms can have more than two relevant levels, and the energy spacing of some of them can be very small (the excited state of alkali atoms is for example split in several levels due to the interaction between the electron and nucleus spins
, separated by a hundred megahertz). This will generate the apparition of other dimples due to these new resonances, but will also create intermediate dimples because atoms having a specific velocity on the x axis will be resonant for one transition with the pump beam and for another transition with the probe beam (thanks again to the Doppler effect).
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
between its ground state and an optically excited state. The accuracy to which these frequencies can be determined should ideally be only limited by the width of the excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
, which is the inverse of the lifetime of this state. However, the samples of atomic gas that are used to that purpose are at room temperature, where the measured frequency distribution is highly broadened due to the Doppler effect
Doppler effect
The Doppler effect , named after Austrian physicist Christian Doppler who proposed it in 1842 in Prague, is the change in frequency of a wave for an observer moving relative to the source of the wave. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from...
. Saturated absorption allows precise spectroscopy of the atomic levels without having to cool the sample down to temperatures where the Doppler broadening is no longer relevant (which would be on the order of a few millikelvins). It is also used to lock the frequency of a laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
to the precise wavelength of an atom in atomic physics experiments.
Doppler broadening of the absorption spectrum of an atom
According to the description of an atom interacting with the electromagnetic fieldElectromagnetic field
An electromagnetic field is a physical field produced by moving electrically charged objects. It affects the behavior of charged objects in the vicinity of the field. The electromagnetic field extends indefinitely throughout space and describes the electromagnetic interaction...
, the atom absorption of light depends on the frequency of the incident field. More precisely, the absorption is characterized by a lorentzian
Lorentzian
Lorentzian may refer to* Cauchy–Lorentz distribution, also known as the Lorentz distribution, Lorentzian function, or Cauchy distribution* Lorentz transformation* Lorentzian inner product* Lorentzian manifoldThe name Lorentz may refer to*Hendrik Lorentz...
of width
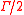 (
( MHz for the atom of Rubidium
MHz for the atom of RubidiumRubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
for example). If we have a cell of atomic vapour at room temperature, then the distribution of velocity will follow a Maxwell–Boltzmann distribution
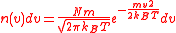
where N is the number of atoms,
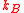 the Boltzmann constant, m the mass of the atom. According to the Doppler effect
the Boltzmann constant, m the mass of the atom. According to the Doppler effectDoppler effect
The Doppler effect , named after Austrian physicist Christian Doppler who proposed it in 1842 in Prague, is the change in frequency of a wave for an observer moving relative to the source of the wave. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from...
formula in the case of non-relativistic speeds
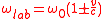
where
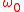 is the frequency of the atom at rest (the one which is being probed). The value of v as a function of
is the frequency of the atom at rest (the one which is being probed). The value of v as a function of 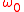 and
and 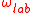 can be inserted in the distribution of velocities. The distribution of absorption as a function of the pulsation will therefore be proportional to a Gaussian of full width at half maximum
can be inserted in the distribution of velocities. The distribution of absorption as a function of the pulsation will therefore be proportional to a Gaussian of full width at half maximumFull width at half maximum
Full width at half maximum is an expression of the extent of a function, given by the difference between the two extreme values of the independent variable at which the dependent variable is equal to half of its maximum value....
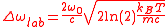
For a Rubidium atom at room temperature,
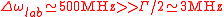
Therefore without any special trick in the experimental setup probing the maximum of absorption of an atomic vapour, the uncertainty of the measurement will be limited by the Doppler broadening and not by the fundamental width of the resonance.
Principle of saturated absorption
To overcome this problem, a classical, and rather general scheme in experimental physics is used, a pump-probe scheme. A laser with a rather high power is sent through the atomic vapor, which represents the pump beam. Another counter-propagating, weak beam is also sent through the atoms, and represents the probe beam ; it has the same frequency as the pump beam. The absorption of the probe beam is recorded on a photodiode for various frequencies of the beams.If the frequency is slightly red-detuned
Laser detuning
In optical physics, laser detuning is the tuning of a laser to a frequency that is slightly off from a quantum system's resonant frequency. Lasers can be detuned in the lab frame so that they are Doppler shifted to the resonant frequency in a moving system, which allows lasers to affect only...
with respect to the atomic frequency, the pump beam will be absorbed by the atoms moving towards its source, the +x direction, while the probe beam will be absorbed by the atoms moving in the -x direction (counter-propagating beams and Doppler effect) which are different from those that interact with the pump beam. On the contrary, if the frequency of the lasers is slightly blue-detuned, the pump beam will be absorbed by the atoms moving away from it in the -x direction, and the probe beam by other atoms moving in the +x direction.
Now let us consider the case of a frequency exactly tuned at the atom frequency. The atoms whose velocity along the x direction is zero will then interact with both the pump and probe beams, which are both resonant with them. Due to its important power, the pump beam will saturate the atomic transition. We can thus consider that in a significant number of cases, a photon coming from the probe beam and arriving on an atom will find it in its excited state. Due to the process of stimulated emission
Stimulated emission
In optics, stimulated emission is the process by which an atomic electron interacting with an electromagnetic wave of a certain frequency may drop to a lower energy level, transferring its energy to that field. A photon created in this manner has the same phase, frequency, polarization, and...
, this photon will desexcite the atom and go through the sample. Thus the probe beam is not completely absorbed at the atomic frequency. On the photodiode, one would then expect to see a Gaussian profile (due to Doppler broadening) with a small dimple in the middle due to the stimulated emission phenomena. This dimple indicates precisely the frequency of the atomic transition.
Experimental realization
As the pump and the probe beam must always have the same frequencies, the most convenient solution is for them to come from the same laser, whose frequency is tuned thanks to current or temperature variations for diode laser for example. The laser is protected from reflections thanks to an optical isolatorOptical isolator
An optical isolator, or optical diode, is an optical component which allows the transmission of light in only one direction. It is typically used to prevent unwanted feedback into an optical oscillator, such as a laser cavity...
. The probe beam can be made of the light reflected by a glass slab (so that its power is much weaker than the power of the pump beam). Some additional modulation can be added either to the laser or to the atoms (thanks for example to a magnetic field) to ease the signal processing afterwards (especially when using a synchronous detection).
The description of the principle of saturated absorption used only a two-level atom. However in real atoms can have more than two relevant levels, and the energy spacing of some of them can be very small (the excited state of alkali atoms is for example split in several levels due to the interaction between the electron and nucleus spins
Hyperfine structure
The term hyperfine structure refers to a collection of different effects leading to small shifts and splittings in the energy levels of atoms, molecules and ions. The name is a reference to the fine structure which results from the interaction between the magnetic moments associated with electron...
, separated by a hundred megahertz). This will generate the apparition of other dimples due to these new resonances, but will also create intermediate dimples because atoms having a specific velocity on the x axis will be resonant for one transition with the pump beam and for another transition with the probe beam (thanks again to the Doppler effect).

