
Sabatier principle
Encyclopedia
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
named after the French chemist Paul Sabatier
Paul Sabatier (chemist)
Paul Sabatier FRS was a French chemist, born at Carcassonne. He taught science classes most of his life before he became Dean of the Faculty of Science at the University of Toulouse in 1905....
. It states that the interactions between the catalyst and the substrate
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
should be "just right"; that is, neither too strong nor too weak. If the interaction is too weak, the substrate will fail to bind to the catalyst and no reaction will take place. On the other hand, if the interaction is too strong, the catalyst gets blocked by substrate or product that fails to dissociate.
The principle can be shown graphically by plotting the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
against a property such as the heat of adsorption of the reactant by the catalyst. Such plots pass through a maximum, looking roughly like a triangle or an inverted parabola, and are called volcano plots because of their shape. Analogous three-dimensional plots can also be built against two different properties, such as the heats of adsorption of the two reactants for a two-component reaction. In that case the plot is generally shown as a contour plot and is called a volcano surface. Volcano plots were introduced by Balandin.
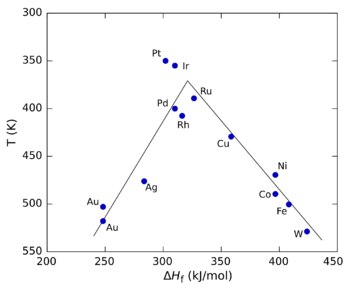
The figure on the right shows a volcano plot for the decomposition of formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
using different transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s as catalysts. In this case, the heat of formation (ΔHf) of the metal formate salt was used for the x axis because studies showed that the reaction intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
was a surface formate. For the y axis, the temperature at which the reaction reaches a specific rate was used (the y axis is plotted in reverse to preserve the conventional "volcano" shape). At low values of ΔHf, the reaction is slow (in other words, requires higher temperatures) because the rate of adsorption is slow and rate-limiting. At high values of ΔHf, desorption becomes the rate-limiting step. The maximum rate, which is observed for the platinum group
Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
metals in this case, requires intermediate values of ΔHf, with the rate being a combination of the rate of adsorption and the rate of desorption.

