
Regular solution
Encyclopedia
In chemistry, a regular solution is a solution that diverges from the behavior of an ideal solution
only moderately. Its entropy of mixing
is equal to that of an ideal solution with the same composition, due to random mixing without strong specific interactions. For two components
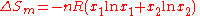
where is the gas constant
is the gas constant
, the total number of moles
the total number of moles
and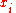 the mole fraction of each component. The enthalpy
the mole fraction of each component. The enthalpy
of mixing is non-zero, unlike for an ideal solution.
A regular solution can also be described by Raoult's law modified with a Margules function
with only one parameter α:
Where the Margules function is
Notice that the Margules function for each component contains the mole fraction of the other component. It can also be shown using the Gibbs-Duhem relation that if the first Margules expression holds, then the other one must have the same shape.
The value of α can be interpreted as W/RT, where W = 2U12 - U11 - U22 represents the difference in interaction energy between like and unlike neighbors.
In contrast to the case of ideal solutions, regular solutions do possess a non-zero enthalpy of mixing, due to the W term. If the unlike interactions are more unfavorable than the like ones, we get competition between an entropy of mixing term that produces a minimum in the Gibbs free energy at x1= 0.5 and the enthalpy term that has a maximum there. At high temperatures the entropy wins and the system is fully miscible, but at lower temperatures the G curve will have two minima and a maximum in between. This results in phase separation. In general there will be a temperature where the three extremes coalesce and the system becomes fully miscible. This point is known as the upper critical solution temperature
or the upper consolute temperature.
In contrast to ideal solutions, the volumes in the case of regular solutions are no longer strictly additive but must be calculated from the partial molar volumes that are a function of x1.
The term was introduced in 1927 by the American physical chemist Joel Henry Hildebrand
.
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
only moderately. Its entropy of mixing
Entropy of mixing
In thermodynamics the entropy of mixing is the increase in the total entropy of a compound system, when different and chemically non-reacting chemical substances or material components are mixed by removing partition between the system's initially separate volumes...
is equal to that of an ideal solution with the same composition, due to random mixing without strong specific interactions. For two components
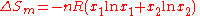
where
 is the gas constant
is the gas constantGas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
,
 the total number of moles
the total number of molesMole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
and
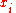 the mole fraction of each component. The enthalpy
the mole fraction of each component. The enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of mixing is non-zero, unlike for an ideal solution.
A regular solution can also be described by Raoult's law modified with a Margules function
Margules function
A Margules function is a function added to the Raoult's law description of a liquid solution to account for deviations from ideality. See also Margules activity model.The amended Raoult's law description of the vapor pressure above the solution becomes:...
with only one parameter α:
- P1= x1P*1f1,M
- P2= x2P*2f2,M
Where the Margules function is
- f1,M = exp(αx22)
- f2,M = exp(αx12)
Notice that the Margules function for each component contains the mole fraction of the other component. It can also be shown using the Gibbs-Duhem relation that if the first Margules expression holds, then the other one must have the same shape.
The value of α can be interpreted as W/RT, where W = 2U12 - U11 - U22 represents the difference in interaction energy between like and unlike neighbors.
In contrast to the case of ideal solutions, regular solutions do possess a non-zero enthalpy of mixing, due to the W term. If the unlike interactions are more unfavorable than the like ones, we get competition between an entropy of mixing term that produces a minimum in the Gibbs free energy at x1= 0.5 and the enthalpy term that has a maximum there. At high temperatures the entropy wins and the system is fully miscible, but at lower temperatures the G curve will have two minima and a maximum in between. This results in phase separation. In general there will be a temperature where the three extremes coalesce and the system becomes fully miscible. This point is known as the upper critical solution temperature
Upper critical solution temperature
The upper critical solution temperature or upper consolute temperature is the critical temperature above which the components of a mixture are miscible in all proportions. The word upper indicates that the UCST is an upper bound to a temperature range of partial miscibility, or miscibility for...
or the upper consolute temperature.
In contrast to ideal solutions, the volumes in the case of regular solutions are no longer strictly additive but must be calculated from the partial molar volumes that are a function of x1.
The term was introduced in 1927 by the American physical chemist Joel Henry Hildebrand
Joel Henry Hildebrand
Joel Henry Hildebrand was an American educator and a pioneer chemist. He was a major figure in chemistry research specializing in liquids and nonelectrolyte solutions.-Education and professorship:...
.

