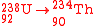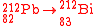Radioactive displacement law of Fajans and Soddy
Encyclopedia
The law of radioactive displacements, also known as Fajans and Soddy law, in radiochemistry
and nuclear physics
, is a rule governing the transmutation of elements
during radioactive decay
. It is named after Frederick Soddy
and Kazimierz Fajans
, who independently arrived at it at about the same time.
The law describes which chemical element
and isotope
is created during the particular type of radioactive decay:
Radiochemistry
Radiochemistry is the chemistry of radioactive materials, where radioactive isotopes of elements are used to study the properties and chemical reactions of non-radioactive isotopes...
and nuclear physics
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
, is a rule governing the transmutation of elements
Nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'...
during radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
. It is named after Frederick Soddy
Frederick Soddy
Frederick Soddy was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also proved the existence of isotopes of certain radioactive elements...
and Kazimierz Fajans
Kazimierz Fajans
-External links:*...
, who independently arrived at it at about the same time.
The law describes which chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
and isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
is created during the particular type of radioactive decay:
- In alpha decayAlpha decayAlpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
, an element is created with an atomic numberAtomic numberIn chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
less by 2 and a mass numberMass numberThe mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
less by four of that of the parent radioisotope, e.g.:
- In beta decayBeta decayIn nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
, the mass number remains unchanged while the atomic number becomes either greater by 1 (β− decay) or less by 1 (β+ decay or electron captureElectron captureElectron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
) from that of the parent radioisotope, e.g.:
See also
- Decay modes in tabular form
- Decay chainDecay chainIn nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...