
Polythionic acid
Encyclopedia
Oxoacid
An oxoacid is an acid that contains oxygen. To be more specific, it is an acid that:#contains oxygen#contains at least one other element#has at least one hydrogen atom bound to oxygen#forms an ion by the loss of one or more protons....
which has a straight chain of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
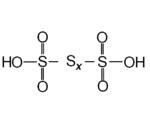
atoms and has the chemical formula H2SnO6 (n > 2). Trithionic acid (H2S3O6), tetrathionic acid (H2S4O6) are simple examples. The compounds of n < 80 are expected to exist, and those of n < 20 have already been synthesized. Dithionic acid
Dithionic acid
Dithionic acid, H2S2O6, is a chemical compound known only in solution.- Salts :Dithionic acid is dibasic and salts called dithionates are known. No acid salts have been discovered. All dithionates are readily soluble in water. They are mild oxidizing and mild reducing agents. The structure of...
(H2S2O6) does not belong to polythionic acid due to the difference in the property.
Stability
Under the condition of strong acidity about pH 1, most of polythionic acids are stable.Synthesis
Various polythionic acids are produced in Wackenroder solution in which sulfur dioxideSulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
and hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
dissolve. The reactions may be as follows although the mechanisms are not clear.
- H2S + H2SO3 → H2S2O2 + H2O
- H2S2O2 + 2H2SO3 → H2S4O6 + 2H2O
- H2S4O6 + H2SO3 → H2S3O6 + H2S2O3
Occurrence
Polythionic acids are often found in crater lakeCrater lake
A crater lake is a lake that forms in a volcanic crater or caldera, such as a maar; less commonly and with lower association to the term a lake may form in an impact crater caused by a meteorite. Sometimes lakes which form inside calderas are called caldera lakes, but often this distinction is not...
s. There are various kind of ions containing sulfur atoms derived by hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and they make the strong acidity condition. It it observed that polythionates in crater lakes are drastically decreased before an eruption occurs. The phenomenon may be useful to predict of volcanic activity.

