Polycarbonate
Overview
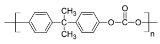
Polycarbonates, known by the trademarked names Lexan
Lexan
Lexan is a registered trademark for SABIC Innovative Plastics' brand of polycarbonate resin thermoplastic. Polycarbonate polymer is produced by reacting bisphenol A with carbonyl dichloride, also known as phosgene. Lexan is the brand name for polycarbonate sheet and resin in a wide range of grades...
, Makrolon, Makroclear
Makroclear
Makroclear is a brand of solid polycarbonate plastic sheet ranging from 0.75 to 15 mm thickness.Polycarbonate is seen as the toughest transparent material...
and others, are a particular group of thermoplastic
Thermoplastic
Thermoplastic, also known as a thermosoftening plastic, is a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently...
polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s. They are easily worked, molded
Injection molding
Injection molding is a manufacturing process for producing parts from both thermoplastic and thermosetting plastic materials. Material is fed into a heated barrel, mixed, and forced into a mold cavity where it cools and hardens to the configuration of the cavity...
, and thermoformed
Thermoforming
Thermoforming is a manufacturing process where a plastic sheet is heated to a pliable forming temperature, formed to a specific shape in a mold, and trimmed to create a usable product...
. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique plastic identification code and are identified as Other, 7.
Polycarbonates received their name because they are polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s containing carbonate group
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
s (–O–(C=O)–O–).

