
Photoinitiator
Encyclopedia
A photoinitiator is any chemical compound that decomposes into free radicals when exposed to light. Photoinitiators are found both in nature (in photochemical smog
) and in industry (for example, in plastics production).
In nature, photoinitiators are present throughout the atmosphere
. For instance, nitrogen dioxide is produced in large quantities by gasoline-burning internal combustion engines. NO2 in the troposphere
gives smog its brown coloration and catalyzes
production of toxic ground-level ozone
. Molecular oxygen (O2) also serves as a photoinitiator in the stratosphere, breaking down into atomic oxygen and in order to form the ozone in the ozone layer.
In industry, photoinitiators are primarily used to promote polymer
ization reactions, notably in the production of polyethylene
plastic
. There are a handful of medical applications as well; for instance, benzoyl peroxide creams are commonly prescribed as acne medication.
Certain azo compounds (such as azobisisobutyronitrile), can also photolytically cleave, forming two alkyl radicals and nitrogen gas:
These free radicals can now promote other reactions.
 Nitrogen dioxide
Nitrogen dioxide
can also be photolytically cleaved by photons of wavelength less than 400 nm producing atomic oxygen and nitric oxide
.
Atomic oxygen is a highly reactive species, and can abstract a H atom from anything, including water.
Nitrogen dioxide can be regenerated through a reaction between certain peroxy-containing radicals and NO.
-production process in the ozone layer
. Oxygen can be photolyzed into atomic oxygen by light with wavelength less than 240 nm.
Atomic oxygen can then combine with more molecular oxygen to form ozone.
However, ozone can also be photolyzed back into O and O2.
Furthermore, atomic oxygen and ozone can combine into O and O3.
This set of reactions govern the production of ozone and can combine to calculate its equilibrium concentration.
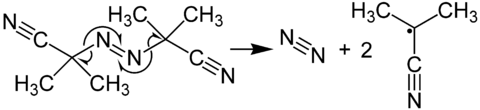
Azobisisobutyronitrile is a white powder often used as a photoinitiator for vinyl-based polymers such as polyvinyl chloride
, also known as PVC. Because this particular photoinitiator produces nitrogen gas (N2) upon decomposition, it is often used as a blowing agent to change the shape and/or texture of plastics.
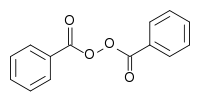
Benzoyl peroxide, much like azobisisobutyronitrile, is a white powder used as a photoinitiator in various commercial and industrial processes, including plastics production. Unlike AIBN, however, benzoyl peroxide produces oxygen gas upon decomposing, giving this compound a host of medical uses as well.
Upon contact with the skin, benzoyl peroxide breaks down, producing oxygen gas, among other things. The oxygen gas is absorbed into the pores of the skin, where it kills off the acne-causing bacteria Propionibacterium acnes
.
In addition, the free radicals produced can break down dead skin cells. Clearing out these dead cells prevents pore blockage and, by extension, acne breakouts.
Smog
Smog is a type of air pollution; the word "smog" is a portmanteau of smoke and fog. Modern smog is a type of air pollution derived from vehicular emission from internal combustion engines and industrial fumes that react in the atmosphere with sunlight to form secondary pollutants that also combine...
) and in industry (for example, in plastics production).
In nature, photoinitiators are present throughout the atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
. For instance, nitrogen dioxide is produced in large quantities by gasoline-burning internal combustion engines. NO2 in the troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
gives smog its brown coloration and catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
production of toxic ground-level ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
. Molecular oxygen (O2) also serves as a photoinitiator in the stratosphere, breaking down into atomic oxygen and in order to form the ozone in the ozone layer.
In industry, photoinitiators are primarily used to promote polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
ization reactions, notably in the production of polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
plastic
Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
. There are a handful of medical applications as well; for instance, benzoyl peroxide creams are commonly prescribed as acne medication.
Reactions
All photoinitiators have bonds that cleave via photolysis. For example, peroxides like hydrogen peroxide are perfect examples, with the O-O bond cleaving to form two hydroxyl radicals.- H2O2 → 2 ·OH
Certain azo compounds (such as azobisisobutyronitrile), can also photolytically cleave, forming two alkyl radicals and nitrogen gas:
- RCH2-N=N-H2CR → 2 RCH2 + N2
These free radicals can now promote other reactions.
Peroxides
Since molecular oxygen can abstract H atoms from certain radicals, the HOO· radical is easily created. This particular radical can further abstract H atoms, creating H2O2, or hydrogen peroxide; peroxides can further cleave photolytically into two hydroxyl radicals. More commonly, HOO can react with free oxygen atoms to yield a hydroxy radical (·OH) and oxygen gas. In both cases, the ·OH radicals formed can serve to oxidize organic compounds in the atmosphere.- H2O2 → 2 ·OH
- HOO· + O → O2 + ·OH
- ·OH + CH4 → ·CH3 + H2O
Nitrogen dioxide

Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
can also be photolytically cleaved by photons of wavelength less than 400 nm producing atomic oxygen and nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
.
- NO2 → NO + O
Atomic oxygen is a highly reactive species, and can abstract a H atom from anything, including water.
- O + H2O → 2 ·OH
Nitrogen dioxide can be regenerated through a reaction between certain peroxy-containing radicals and NO.
- ROO· + NO → NO2 + RO·
Molecular oxygen
In the stratosphere, molecular oxygen (O2) is an important photoinitiator that begins the ozoneOzone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
-production process in the ozone layer
Ozone layer
The ozone layer is a layer in Earth's atmosphere which contains relatively high concentrations of ozone . This layer absorbs 97–99% of the Sun's high frequency ultraviolet light, which is potentially damaging to the life forms on Earth...
. Oxygen can be photolyzed into atomic oxygen by light with wavelength less than 240 nm.
- O2 → 2O
Atomic oxygen can then combine with more molecular oxygen to form ozone.
- O + O2 → O3
However, ozone can also be photolyzed back into O and O2.
- O3 → O + O2
Furthermore, atomic oxygen and ozone can combine into O and O3.
- O + O3 → 2 O2
This set of reactions govern the production of ozone and can combine to calculate its equilibrium concentration.
AIBN

Azobisisobutyronitrile is a white powder often used as a photoinitiator for vinyl-based polymers such as polyvinyl chloride
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
, also known as PVC. Because this particular photoinitiator produces nitrogen gas (N2) upon decomposition, it is often used as a blowing agent to change the shape and/or texture of plastics.
Benzoyl peroxide

Benzoyl peroxide, much like azobisisobutyronitrile, is a white powder used as a photoinitiator in various commercial and industrial processes, including plastics production. Unlike AIBN, however, benzoyl peroxide produces oxygen gas upon decomposing, giving this compound a host of medical uses as well.
Upon contact with the skin, benzoyl peroxide breaks down, producing oxygen gas, among other things. The oxygen gas is absorbed into the pores of the skin, where it kills off the acne-causing bacteria Propionibacterium acnes
Propionibacterium acnes
Propionibacterium acnes is a relatively slow growing, typically aerotolerant anaerobic gram positive bacterium that is linked to the skin condition acne; it can also cause chronic blepharitis and endophthalmitis, the latter particularly following intraocular surgery...
.
In addition, the free radicals produced can break down dead skin cells. Clearing out these dead cells prevents pore blockage and, by extension, acne breakouts.

