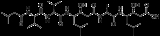
Pepstatin
Encyclopedia
Pepstatin is a potent inhibitor of aspartyl proteases. It is a hexa-peptide
containing the unusual amino acid statine
(Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid), having the sequence Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta-Ala-Sta) . It was originally isolated from cultures of various species of Actinomyces
due to its ability to inhibit pepsin
at picomolar concentrations. It was later found to inhibit nearly all acid proteases with high potency and, as such, has become a valuable research tool, as well as a common constituent of protease inhibitor cocktails.
Pepstatin is practically insoluble in water, chloroform, ether, and benzene, however it can be dissolved in methanol, ethanol and DMSO with acetic acid, to between 1 and 5 mg/ml.
]
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
containing the unusual amino acid statine
Statine
Statine is a gamma amino acid that occurs twice in the sequence of pepstatin, a protease inhibitor that is active against pepsin and other acid proteases. It is thought to be responsible for the inhibitory activity of pepstatin because it mimics the tetrahedral transition state of peptide catalysis....
(Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid), having the sequence Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta-Ala-Sta) . It was originally isolated from cultures of various species of Actinomyces
Actinomyces
Actinomyces from Greek "actino" that means mucus and fungus, is a genus of the actinobacteria class of bacteria. They are all Gram-positive and are characterized by contiguous spread, suppurative and granulomatous inflammation, and formation of multiple abscesses and sinus tracts that may...
due to its ability to inhibit pepsin
Pepsin
Pepsin is an enzyme whose precursor form is released by the chief cells in the stomach and that degrades food proteins into peptides. It was discovered in 1836 by Theodor Schwann who also coined its name from the Greek word pepsis, meaning digestion...
at picomolar concentrations. It was later found to inhibit nearly all acid proteases with high potency and, as such, has become a valuable research tool, as well as a common constituent of protease inhibitor cocktails.
Pepstatin is practically insoluble in water, chloroform, ether, and benzene, however it can be dissolved in methanol, ethanol and DMSO with acetic acid, to between 1 and 5 mg/ml.
]

