
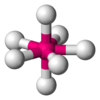
Pentagonal bipyramid molecular geometry
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a pentagonal bipyramid (or dipyramid) is a molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
with one atom at the centre with seven ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s at the corners of a pentagonal dipyramid
Pentagonal dipyramid
In geometry, the pentagonal bipyramid is third of the infinite set of face-transitive bipyramids.Each bipyramid is the dual of a uniform prism.If the faces are equilateral triangles, it is a deltahedron and a Johnson solid...
. A perfect pentagonal bipyramid belongs to the molecular point group D5h .
The pentagonal bipyramid is a case where bond angles surrounding an atom are not identical (see also Trigonal bipyramid molecular geometry
Trigonal bipyramid molecular geometry
In chemistry a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular dipyramid...
). Other seven coordinate geometries include the mono-capped octahedron and mono-capped trigonal prism. A variety of transition metal complexes adopt heptacoordination, but the symmetry is usually lower than D5h.
Examples
- Iodine heptafluorideIodine heptafluorideIodine heptafluoride, also known as iodine fluoride or even iodine fluoride, is an interhalogen compound with chemical formula IF7. It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory...
(IF7) with 7 bonding groups - Peroxo Cr(IV)-complexes, e.g. [Cr(O2)2(NH3)3] where the peroxo groups occupy four of the planar positions.
External links
- http://www.ch.ic.ac.uk/rzepa/bpr/Figure-5.html - Images of IF7
- 3D Chem - Chemistry, Structures, and 3D Molecules
- IUMSC - Indiana University Molecular Structure Center

