
Paleothermometer
Encyclopedia
A paleothermometer is a methodology for determining past temperature
s using a proxy
found in a natural record such as a sediment
, ice core
, tree rings or TEX86.
The ratio of 18O to 16O, usually in foram tests or ice cores. High values mean low temperatures. Confounded by ice volume - more ice means higher values.
Ocean water is mostly H216O, with small amounts of HD16O and H218O. In Standard Mean Ocean Water (SMOW) the ratio of D to H is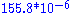 and 18O/16O is
and 18O/16O is 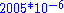 . Fractionation occurs during changes between condensed and vapour phases: the vapour pressure of heavier isotopes is lower, so vapour contains relatively more of the lighter isotopes and when the vapour condenses the precipitation preferentially contains heavier isotopes. The difference from SMOW is expressed as δ18
. Fractionation occurs during changes between condensed and vapour phases: the vapour pressure of heavier isotopes is lower, so vapour contains relatively more of the lighter isotopes and when the vapour condenses the precipitation preferentially contains heavier isotopes. The difference from SMOW is expressed as δ18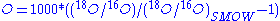 ; and a similar formula for δD. values for precipitation are always negative. The major influence on is the difference between ocean temperatures where the moisture evaporated and the place where the final precipitation occurred; since ocean temperatures are relatively stable the value mostly reflects the temperature where precipitation occurs. Taking into account that the precipitation forms above the inversion layer
; and a similar formula for δD. values for precipitation are always negative. The major influence on is the difference between ocean temperatures where the moisture evaporated and the place where the final precipitation occurred; since ocean temperatures are relatively stable the value mostly reflects the temperature where precipitation occurs. Taking into account that the precipitation forms above the inversion layer
, we are left with a linear relation:
which is empirically calibrated from measurements of temperature and as a = 0.67 ‰/oC for Greenland
and 0.76 ‰/oC for East Antarctica. The calibration was initially done on the basis of spatial variations in temperature and it was assumed that this corresponded to temporal variations (Jouzel and Merlivat, 1984). More recently, borehole thermometry has shown that for glacial-interglacial variations, a = 0.33 ‰/oC (Cuffey et al., 1995), implying that glacial-interglacial temperature changes were twice as large as previously believed.
; higher temperatures make it easier to incorporate. Therefore a high Mg/Ca ratio implies a high temperature, although ecological factors may confound the signal. Mg has a long residence time
in the ocean, and so it is possible to largely ignore the effect of changes in seawater Mg/Ca on the signal.
Strontium (Sr) incorporates in coral aragonite, and it is well established that the precise Sr/Ca ratio in the coral skeleton shows an inverse correlation with the seawater temperature during its biomineralization.
and photosynthesis
. Quantitative analyses of modern vegetation leaf physiognomy and climate responses along environmental gradients have been largely univariate
, but multivariate approaches integrate multiple leaf characters and climatic parameters. Temperature has been estimated (to varying degrees of fidelity) using leaf physiognomy for Late Cretaceous
and Cenozoic
leaf floras, principally using two main approaches:
approach that is based on the observation that the proportion of woody dicot species with smooth (i.e. non-toothed) leaf margins (0 ≥ Pmargin ≥ 1) in vegetation varies proportionately with mean annual temperature (MAT). Requires the fossil flora to be segregated into morphotypes (i.e. ‘species’), but does not require their identification. The original LMA regression equation was derived for East Asian forests, and is: MAT = 1.141 +(0.306 * Pmargin), standard error ± 2.0°C
The error of the estimate for LMA is expressed as the binomial sampling error: σ[LMA] = c √(Pmargin (1 - Pmargin) / r
where c is the slope from the LMA regression equation, Pmargin as used in (1), and r is the number of species scored for leaf margin type for the individual fossil leaf flora.
Alternative LMA calibrations have been derived for major world regions, including North America, Europe, South America, and Australia.
or random distribution of the same concentration of isotopes. The excess is greatest at low temperature (see Van 't Hoff equation), with the isotopic distribution becoming more randomized at higher temperature. Along with the closely related phenomenon of equilibrium isotope fractionation
, this effect arises from differences in zero point energy among isotopologues. Carbonate minerals like calcite contain CO32– groups that can be converted to CO2 gas by reaction with concentrated phosphoric acid. The CO2 gas is analyzed with a mass spectrometer, to determine the abundances of isotopologues. The parameter Δ47 is the measured difference in concentration between isotopologue
s with a mass of 47 u
(as compared to 44) in a sample and a hypothetical sample with the same bulk isotopic composition, but a stochastic
distribution of heavy isotopes. Lab experiments, quantum mechanical calculations, and natural samples (with known crystallization temperatures) all indicate that Δ47 is correlated to the inverse square of temperature
. Thus Δ47 measurements provide an estimation of the temperature at which a carbonate formed. 13C-18O paleothermometry does not require prior knowledge of the concentration of 18O in the water (which the δ18O method does). This allows the 13C-18O paleothermometer to be applied to some samples, including freshwater carbonates and very old rocks, with less ambiguity than other isotope-based methods. The method is presently limited by the very low concentration of isotopologues of mass 47 or higher in CO2 produced from natural carbonates, and by the scarcity of instruments with appropriate detector arrays and sensitivities. The study of these types of isotopic ordering reactions in nature is often called "clumped-isotope" geochemistry.
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
s using a proxy
Proxy (climate)
In the study of past climates is known as paleoclimatology, climate proxies are preserved physical characteristics of the past that stand in for direct measurements , to enable scientists to reconstruct the climatic conditions that prevailed during much of the Earth's history...
found in a natural record such as a sediment
Sediment
Sediment is naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of fluids such as wind, water, or ice, and/or by the force of gravity acting on the particle itself....
, ice core
Ice core
An ice core is a core sample that is typically removed from an ice sheet, most commonly from the polar ice caps of Antarctica, Greenland or from high mountain glaciers elsewhere. As the ice forms from the incremental build up of annual layers of snow, lower layers are older than upper, and an ice...
, tree rings or TEX86.
The ratio of 18O to 16O, usually in foram tests or ice cores. High values mean low temperatures. Confounded by ice volume - more ice means higher values.
Ocean water is mostly H216O, with small amounts of HD16O and H218O. In Standard Mean Ocean Water (SMOW) the ratio of D to H is
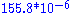 and 18O/16O is
and 18O/16O is 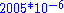 . Fractionation occurs during changes between condensed and vapour phases: the vapour pressure of heavier isotopes is lower, so vapour contains relatively more of the lighter isotopes and when the vapour condenses the precipitation preferentially contains heavier isotopes. The difference from SMOW is expressed as δ18
. Fractionation occurs during changes between condensed and vapour phases: the vapour pressure of heavier isotopes is lower, so vapour contains relatively more of the lighter isotopes and when the vapour condenses the precipitation preferentially contains heavier isotopes. The difference from SMOW is expressed as δ18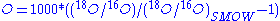 ; and a similar formula for δD. values for precipitation are always negative. The major influence on is the difference between ocean temperatures where the moisture evaporated and the place where the final precipitation occurred; since ocean temperatures are relatively stable the value mostly reflects the temperature where precipitation occurs. Taking into account that the precipitation forms above the inversion layer
; and a similar formula for δD. values for precipitation are always negative. The major influence on is the difference between ocean temperatures where the moisture evaporated and the place where the final precipitation occurred; since ocean temperatures are relatively stable the value mostly reflects the temperature where precipitation occurs. Taking into account that the precipitation forms above the inversion layerInversion (meteorology)
In meteorology, an inversion is a deviation from the normal change of an atmospheric property with altitude. It almost always refers to a temperature inversion, i.e...
, we are left with a linear relation:
- δ18O = aT + b
which is empirically calibrated from measurements of temperature and as a = 0.67 ‰/oC for Greenland
Greenland
Greenland is an autonomous country within the Kingdom of Denmark, located between the Arctic and Atlantic Oceans, east of the Canadian Arctic Archipelago. Though physiographically a part of the continent of North America, Greenland has been politically and culturally associated with Europe for...
and 0.76 ‰/oC for East Antarctica. The calibration was initially done on the basis of spatial variations in temperature and it was assumed that this corresponded to temporal variations (Jouzel and Merlivat, 1984). More recently, borehole thermometry has shown that for glacial-interglacial variations, a = 0.33 ‰/oC (Cuffey et al., 1995), implying that glacial-interglacial temperature changes were twice as large as previously believed.
Mg/Ca and Sr/Ca
Magnesium (Mg) can be incorporated into the shells (tests) of planktic and benthic foraminiferaForaminifera
The Foraminifera , or forams for short, are a large group of amoeboid protists which are among the commonest plankton species. They have reticulating pseudopods, fine strands of cytoplasm that branch and merge to form a dynamic net...
; higher temperatures make it easier to incorporate. Therefore a high Mg/Ca ratio implies a high temperature, although ecological factors may confound the signal. Mg has a long residence time
Residence time
Residence time is the average amount of time that a particle spends in a particular system. This measurement varies directly with the amount of substance that is present in the system....
in the ocean, and so it is possible to largely ignore the effect of changes in seawater Mg/Ca on the signal.
Strontium (Sr) incorporates in coral aragonite, and it is well established that the precise Sr/Ca ratio in the coral skeleton shows an inverse correlation with the seawater temperature during its biomineralization.
Alkenones
Distributions of organic molecules in marine sediments reflect temperature.Leaf physiognomy
The characteristic leaf sizes, shapes and prevalence of features such as drip tips (‘leaf or foliar physiognomy’) differs between tropical rainforests (many species with large leaves with smooth edges and drip tips) and temperate deciduous forests (smaller leaf size classes common, toothed edges common), and is often continuously variable between sites along climatic gradients, such as from hot to cold climates, or high to low precipitation. This variation between sites along environmental gradients reflects adaptive compromises by the species present to balance the need to capture light energy, manage heat gain and loss, while maximising the efficiency of gas exchange, transpirationTranspiration
Transpiration is a process similar to evaporation. It is a part of the water cycle, and it is the loss of water vapor from parts of plants , especially in leaves but also in stems, flowers and roots. Leaf surfaces are dotted with openings which are collectively called stomata, and in most plants...
and photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
. Quantitative analyses of modern vegetation leaf physiognomy and climate responses along environmental gradients have been largely univariate
Univariate
In mathematics, univariate refers to an expression, equation, function or polynomial of only one variable. Objects of any of these types but involving more than one variable may be called multivariate...
, but multivariate approaches integrate multiple leaf characters and climatic parameters. Temperature has been estimated (to varying degrees of fidelity) using leaf physiognomy for Late Cretaceous
Late Cretaceous
The Late Cretaceous is the younger of two epochs into which the Cretaceous period is divided in the geologic timescale. Rock strata from this epoch form the Upper Cretaceous series...
and Cenozoic
Cenozoic
The Cenozoic era is the current and most recent of the three Phanerozoic geological eras and covers the period from 65.5 mya to the present. The era began in the wake of the Cretaceous–Tertiary extinction event at the end of the Cretaceous that saw the demise of the last non-avian dinosaurs and...
leaf floras, principally using two main approaches:
Leaf margin analysis
A univariateUnivariate
In mathematics, univariate refers to an expression, equation, function or polynomial of only one variable. Objects of any of these types but involving more than one variable may be called multivariate...
approach that is based on the observation that the proportion of woody dicot species with smooth (i.e. non-toothed) leaf margins (0 ≥ Pmargin ≥ 1) in vegetation varies proportionately with mean annual temperature (MAT). Requires the fossil flora to be segregated into morphotypes (i.e. ‘species’), but does not require their identification. The original LMA regression equation was derived for East Asian forests, and is: MAT = 1.141 +(0.306 * Pmargin), standard error ± 2.0°C
The error of the estimate for LMA is expressed as the binomial sampling error: σ[LMA] = c √(Pmargin (1 - Pmargin) / r
where c is the slope from the LMA regression equation, Pmargin as used in (1), and r is the number of species scored for leaf margin type for the individual fossil leaf flora.
Alternative LMA calibrations have been derived for major world regions, including North America, Europe, South America, and Australia.
CLAMP (Climate leaf analysis multivariate program)
CLAMP is a multivariate approach largely based on a data set of primarily western hemisphere vegetation, subsequently added to with datasets from additional world regional vegetation. Canonical Correlation Analysis is used combining 31 leaf characters, but leaf margin type represented a significant component of the relationship between physiognomic states and temperature. Using CLAMP, MAT is estimated with small standard errors (e.g. CCA ± 0.7–1.0°C). Additional temperature parameters can be estimated using CLAMP, such as the coldest month mean temperature (CMMT) and the warmest month mean temperature (WMMT) which provide estimates for winter and summer mean conditions respectively.Nearest living relative analogy / coexistence analysis
Certain plants prefer certain temperatures; if their pollen is found one can work out the approximate temperature.13C-18O bonds in carbonates
There is a slight thermodynamic tendency for heavy isotopes to form bonds with each other, in excess of what would be expected from a stochasticStochastic
Stochastic refers to systems whose behaviour is intrinsically non-deterministic. A stochastic process is one whose behavior is non-deterministic, in that a system's subsequent state is determined both by the process's predictable actions and by a random element. However, according to M. Kac and E...
or random distribution of the same concentration of isotopes. The excess is greatest at low temperature (see Van 't Hoff equation), with the isotopic distribution becoming more randomized at higher temperature. Along with the closely related phenomenon of equilibrium isotope fractionation
Equilibrium fractionation
Equilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. Equilibrium fractionation is strongest at low temperatures, and forms the basis of the most widely used isotopic paleothermometers : D/H and 18O/16O records from ice...
, this effect arises from differences in zero point energy among isotopologues. Carbonate minerals like calcite contain CO32– groups that can be converted to CO2 gas by reaction with concentrated phosphoric acid. The CO2 gas is analyzed with a mass spectrometer, to determine the abundances of isotopologues. The parameter Δ47 is the measured difference in concentration between isotopologue
Isotopologue
Isotopologues are molecules that differ only in their isotopic composition. Simply, the isotopologue of a chemical species has at least one atom with a different number of neutrons than the parent....
s with a mass of 47 u
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
(as compared to 44) in a sample and a hypothetical sample with the same bulk isotopic composition, but a stochastic
Stochastic
Stochastic refers to systems whose behaviour is intrinsically non-deterministic. A stochastic process is one whose behavior is non-deterministic, in that a system's subsequent state is determined both by the process's predictable actions and by a random element. However, according to M. Kac and E...
distribution of heavy isotopes. Lab experiments, quantum mechanical calculations, and natural samples (with known crystallization temperatures) all indicate that Δ47 is correlated to the inverse square of temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. Thus Δ47 measurements provide an estimation of the temperature at which a carbonate formed. 13C-18O paleothermometry does not require prior knowledge of the concentration of 18O in the water (which the δ18O method does). This allows the 13C-18O paleothermometer to be applied to some samples, including freshwater carbonates and very old rocks, with less ambiguity than other isotope-based methods. The method is presently limited by the very low concentration of isotopologues of mass 47 or higher in CO2 produced from natural carbonates, and by the scarcity of instruments with appropriate detector arrays and sensitivities. The study of these types of isotopic ordering reactions in nature is often called "clumped-isotope" geochemistry.

