
Oxidation of secondary alcohols to ketones
Encyclopedia
The oxidation of secondary alcohols to ketones is an important oxidation
reaction in organic chemistry
.
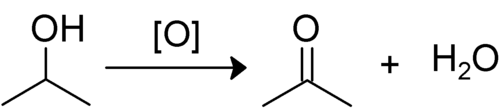 When a secondary alcohol is oxidised, it is converted to a ketone
When a secondary alcohol is oxidised, it is converted to a ketone
. The hydrogen from the hydroxyl
group is lost along with the hydrogen bonded to the second carbon. The remaining oxygen then double bonds with the carbon. This leaves a ketone, as R1-CO-R2. Ketones cannot normally be oxidised any further because this would involve breaking a C-C bond, which requires too much energy .
The reaction can occur using a variety of oxidants.
.
The orange-red dichromate ion, Cr2O72-, is reduced to the green Cr3+ ion. This reaction was once used in an alcohol breath test.
is a mild oxidant for the conversion of alcohols to aldehydes or ketones.
The reaction is performed under standard conditions, at room temperature, with either dichloromethane
or trichloromethane. The reaction takes between half an hour and two hours to complete. The products are then separated from the byproduct, an iodide compound.
and dimethylsulfoxide. It also produces an organic base, such as triethylamine
.
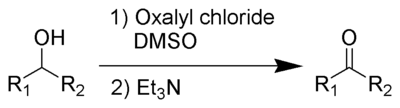 The by-products are dimethyl sulfide
The by-products are dimethyl sulfide
(Me2S), carbon monoxide
(CO), carbon dioxide
(CO2) and - when triethylamine is used as base — triethylammonium chloride (C6H15NHCl). Dimethyl sulfide and carbon monoxide are very toxic and volatile compounds, so the reaction and the work-up needs to be performed in a fume cupboard.
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.

Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. The hydrogen from the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group is lost along with the hydrogen bonded to the second carbon. The remaining oxygen then double bonds with the carbon. This leaves a ketone, as R1-CO-R2. Ketones cannot normally be oxidised any further because this would involve breaking a C-C bond, which requires too much energy .
The reaction can occur using a variety of oxidants.
Potassium dichromate
A secondary alcohol can be oxidised into a ketone using acidified potassium dichromate and heating under refluxReflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
.
The orange-red dichromate ion, Cr2O72-, is reduced to the green Cr3+ ion. This reaction was once used in an alcohol breath test.
Dess-Martin oxidation
The Dess-Martin ReagentDess-Martin periodinane
Dess–Martin periodinane is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions , shorter reaction times, higher yields, simplified workups,...
is a mild oxidant for the conversion of alcohols to aldehydes or ketones.
The reaction is performed under standard conditions, at room temperature, with either dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
or trichloromethane. The reaction takes between half an hour and two hours to complete. The products are then separated from the byproduct, an iodide compound.
Swern oxidation
Swern oxidation oxidises secondary alcohols into ketones using oxalyl chlorideOxalyl chloride
Oxalyl chloride or ethanedioyl dichloride is a chemical compound with the formula 2. This colourless, sharp-smelling liquid, the diacid chloride of oxalic acid, is a useful reagent in organic synthesis...
and dimethylsulfoxide. It also produces an organic base, such as triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
.

Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
(Me2S), carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(CO), carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) and - when triethylamine is used as base — triethylammonium chloride (C6H15NHCl). Dimethyl sulfide and carbon monoxide are very toxic and volatile compounds, so the reaction and the work-up needs to be performed in a fume cupboard.

