Nocaine
Encyclopedia
- "Nocaine" redirects here, and is not to be confused with "NorcocaineNorcocaineNorcocaine is a minor metabolite of cocaine. It is the only confirmed pharmacologically active metabolite of cocaine, although salicylmethylecgonine is also speculated to be an active metabolite....
"
(+)-CPCA (nocaine, 3α-carbomethoxy-4β-(4-chlorophenyl)-N-methylpiperidine) is a stimulant
Stimulant
Stimulants are psychoactive drugs which induce temporary improvements in either mental or physical function or both. Examples of these kinds of effects may include enhanced alertness, wakefulness, and locomotion, among others...
drug similar in structure to RTI-31
RTI-31
-2β-Carbomethoxy-3β-tropane is a synthetic analog of cocaine that acts as a stimulant. Semi-synthesis of this compound is dependent upon the availability of cocaine starting material. According to the article, RTI-31 is 64 x the strength of cocaine in terms of its potency to elicit...
, but lacking the two-carbon bridge of the tropane
Tropane
Tropane is a nitrogenous bicyclic organic compound. It is mainly known for a group of alkaloids derived from it , which include, among others, atropine and cocaine. Both alkaloids contain tropinone from which tropane is a derivate...
skeleton . This compound was first developed as a substitute agent for cocaine.
Since this time a large number of substituted phenylpiperidine
Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
derivatives have been discovered, hybridizing the basic nocaine structure with that of other similar molecules such as methylphenidate
Methylphenidate
Methylphenidate is a psychostimulant drug approved for treatment of attention-deficit hyperactivity disorder, postural orthostatic tachycardia syndrome and narcolepsy. It may also be prescribed for off-label use in treatment-resistant cases of lethargy, depression, neural insult and obesity...
, meperidine and modafinil
Modafinil
Modafinil is an analeptic drug manufactured by Cephalon, and is approved by the U.S. Food and Drug Administration for the treatment of narcolepsy, shift work sleep disorder, and excessive daytime sleepiness associated with obstructive sleep apnea...
to create a large family of derivatives with a range of activity profiles and potential applications. This is a significant field of research with much work ongoing, and dozens of novel compounds have been developed although none have yet come to market.
The Nocaine family includes a diverse assortment of piperidine based cocaine mimics. The parent compound Nocaine was developed in an attempt to develop a substitute drug for cocaine
Cocaine
Cocaine is a crystalline tropane alkaloid that is obtained from the leaves of the coca plant. The name comes from "coca" in addition to the alkaloid suffix -ine, forming cocaine. It is a stimulant of the central nervous system, an appetite suppressant, and a topical anesthetic...
for the treatment of addiction, and was found to substitute for cocaine in animal models while having significantly less abuse potential itself.
Routes of synthesis
To make any of the phenyltropanes requires either a source of cocaine, or extensive and repeated separation of enantiomers due to the lack of enantioselective routes to the essential intermediate methylecgonidineMethylecgonidine
Methylecgonidine is a chemical intermediate derived from ecgonine or cocaine.Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form...
and the large differences in potency between different structural isomers of the final product.
Laboratory synthesis has been devised but is hampered by the fact that in addition to the wanted isomer of anhydroecgonidine, they are also saddled with the unwanted enantiomer.
Basic Pharmacology
Like cocaine, (–)-cis-CPCA and (+)-CPCA bind to the dopamine transporterDopamine transporter
The dopamine transporter is a membrane-spanning protein that pumps the neurotransmitter dopamine out of the synapse back into cytosol, from which other transporters sequester DA and NE into vesicles for later storage and release...
and inhibit dopamine
Dopamine
Dopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this substituted phenethylamine functions as a neurotransmitter, activating the five known types of dopamine receptors—D1, D2, D3, D4, and D5—and their...
uptake, stimulate motor activity in rodents and completely substitute for cocaine in discrimination tests. Pretreatment with (–)-cis-CPCA or (+)-CPCA enhances the cocaine discriminative stimulus in rats. However there are a number of differences; the locomotor stimulant effects of the piperidine derivatives are much less than those induced by cocaine, and pretreating mice with (–)-cis-CPCA or (+)-CPCA does not increase cocaine induced convulsions, and actually reduced cocaine induced locomotor stimulation. The (–)-cis-CPCA isomer has similar reinforcing effects to cocaine as shown by fixed-ratio self-administration tests in rats, but (+)-CPCA has a flat dose-response curve, and similarly while (–)-cis-CPCA and cocaine had nearly identical break points in a "punished responding" (?) self administration test, (+)-CPCA had a lower break point than either of the other drugs.
| Monoamine Reuptake Activity (nM) | |||
| Compound | [3H]NE | [3H]5-HT | [3H]DA |
| Cocaine | 119 | 177 | 275 |
| (–)-cis-CPCA | 98 | 390 | 67 |
| (+)-CPCA | 90 | 5900 | 276 |
The generally lower efficacy of (+)-CPCA in locomotor and methamphetamine discrimination tests could result from the differential selectivity of the two isomers for the DAT relative to the SERT. That is, if serotonin receptor activation is requisite for maximal efficacy, the difference SERT affinity between (–)-cis-CPCA and (+)-CPCA might play a contributory role in accounting for the differences in the observed pharmacology. Catecholamine selective drugs, like TMP (methylphenidate), are reported to possess decent abuse potential though, so it is not easy to gauge why (+)-CPCA does not entice a strong self-administration propensity.
A possible explanation might be nocaine preferentially binds to the ↓ DAT, in which case it would be expected to behave somewhat differently to cocaine. Some sort of cholinergic effect might also be aversive. For example, muscarinic activity of benztropine analogs is known to limit their reinforcing potential. Ion-channel activity is another factor that can be used to explain certain differences in pharmacology.
It is possible that sigma receptor
Sigma receptor
The sigma receptors σ1 and σ2 bind to ligands such as 4-PPBP, SA 4503, ditolylguanidine, dimethyltryptamine and siramesine.- Classification :...
activity might also account for some of the differences between cocaine and these piperidine mimics (R. Matsumoto, et al. 2001, (Ping and Teruo, 2003 rev). Sigma receptors are not specific to cocaine, other psychostimulants like methamphetamine (E. Nguyen, et al. 2005), and phencyclidine are also linked to this neural target. An increased understanding of this receptor recently led to a novel AD being reported that is based around its pharmacology.
In summary, (+)-CPCA has lower potency and efficacy than cocaine in increasing locomotor activity in rodents. (+)-CPCA only manages to produce partial methamphetamine-like discriminative stimulus effects, although it is fully cocaine-like in cocaine-trained animals. (+)-CPCA has lower reinforcing potential than cocaine as assessed by fixed and progressive ratio IV self-administration tests in rats, with its reinforcing effects confirmed by rhesus monkeys. Furthermore, (+)-CPCA dose dependently antagonizes cocaine-induced locomotion and potentiates the discriminative stimulus effects of a low dose of cocaine. (+)-CPCA, unlike cocaine, does not enhance cocaine-induced convulsions. These results suggest that (+)-CPCA completely mimics certain behavioral actions of cocaine, whereas acting like a weak partial agonist in others, including its ability to attenuate cocaine-induced increase in locomotion and to serve as a positive reinforcing agent in rodents. Thus, (+)-CPCA may have potential utility in the treatment of cocaine addiction, and also offer valuable pharmacological information, furthering our understanding of cocaines mechanism of action, because it exhibits fundamental differences from other related DARI molecules.
Nocaine: Ester and Amine Modifications
A series of novel N- and 3α-modified Nocaine analogs were synthesized and tested for their SNDRI activity and behavioral properties in mice.The rational design of ligands with a predetermined potency at and selectivity for monoamine transporters is hindered by the lack of knowledge about the 3D structure of these targets. In cases where the 3D structure of the binding site in a target protein is not well defined, as is the case for the monoamine transporter
Monoamine transporter
Monoamine transporters are protein structures that function as integral plasma membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. Three major classes of MATs are responsible for the reuptake of their associated amine neurotransmitters...
proteins, one can perform ligand-based design to develop a pharmacophore
Pharmacophore
thumb|right|300px|An example of a pharmacophore model.A pharmacophore is an abstract description of molecular features which are necessary for molecular recognition of a ligand by a biological macromolecule....
. That is, by studying the conformational properties of a series of pharmacologically similar compounds, one can form hypotheses regarding the pharmacophore. Most of the potent tropane-based inhibitors, inc. coca, are believed to have at least 3 major interactions with the transporter binding site: one ionic or H-bonding interaction at the basic nitrogen, one dipole-dipole or H-bonding interaction of the ester group, and an interaction of the aryl group with a lipophilic binding pocket. This model was successfully used for the design of a novel piperidine-based DAT inhibitor, that is economically affordable to manufacture.
Although the in vivo metabolism of (+)-CPCA is also likely to involve N-demethylation, metabolism to the corresponding free acid, to give a compound inactive at all monoamine transporters, will probably be the predominant pathway in vivo. It was reasoned that metabolism via esterase action can be avoided by replacing the ester group with a bioisosteric group that is more stable to metabolic degradation. In previous studies, it was found that oxadiazole, although cocaine-like in activity, exhibits a significantly longer duration of action due to slower rate of metabolism. In general, relative to the corresponding N-methyl compounds, the norpiperidines exhibited an increased activity at the SERT/NET and only modest changes at the DAT.
| Ki (nM) | |||
| R | NE | DA | 5HT |
| CO2Me | 252 → 7.9 | 233 → 279 | 8490 → 434 |
| CH2OH | 198 → 69 | 497 → 836 | 1550 → 239 |
| Oxadiazole | 256 → 34 | 187 → 189 | 5960 → 373 |
An interesting difference between cocaine, ester 1a, alcohol 2a, and norester 1b is that the latter two compounds are substantially longer acting than cocaine in locomotor activity tests in mice. Although prolonged action is anticipated from compounds like alcohol 2a and oxadiazole 3a which lack the 3α ester group and so are more difficult to metabolise, this is not expected for the norester 1b, because the 3α ester group should be just as easily hydrolysed as the ester group of cocaine and 1a. Another result of N-demethylation is an initial depressant action of 1b followed by delayed locomotor stimulation, which might be due to interaction with GABA receptors or mGlu5
Metabotropic glutamate receptor 5
Metabotropic glutamate receptor 5 is a protein that in humans is encoded by the GRM5 gene.- Function :The amino acid L-glutamate is the major excitatory neurotransmitter in the central nervous system and activates both ionotropic and metabotropic glutamate receptors...
.
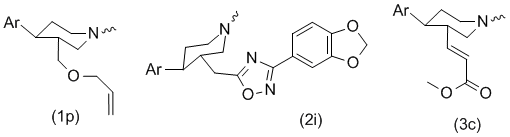
3α-Substituted Nocaine Ligand Design
In an earlier study, it was found that 3α-amido and bulky 3α-oxadiazoyl nocaine ligands, which possess greater stability relative to the ester functional group, and are therefore more attractive as potential therapies, are inactive. This result led to the hypothesis that the binding site of the DAT and NET in close proximity to the 3α-position of the piperidine ring is compact and cannot accommodate bulky, sterically occluded substituents, like the 3-substituted 1,2,4-oxadiazolyl groups. Supplied with this information, it was reasoned that introduction of a methylene spacer would confer improved monoamine transporter binding affinity upon the resultant molecules.| R | [3H]DA | [3H]5-HT | [3H]NE |
| CO2Me | 233 | 8490 | 252 |
| CONMe2 | 2140 | 18900 | 569 |
| CH2OAc | 599 | 901 | 235 |
| CH2OCH2CH=CH2 | 60 | 231 | 20 |
| CH2CO2Et | 79 | 191 | 101 |
| CH2CONMe2 | 16 | 1994 | 46 |
| Heterocycle | 44 | 32 | 52 |
| CH2CH2CO2Me | 68 | 255 | 31 |
| trans-CH=CHCO2Me | 53 | 501 | 272 |
| Prn | 20 | 228 | 6.5 |
| (CH2)3OH | 16 | 2810 | 564 |
One of the possible reasons that the C2–C3 compounds are more active than the C1 compounds is that the polar group present in the more flexible 3α-appendage of the C2–C3 ligands is able to avoid unfavorable interactions with the binding site in close proximity to the piperidine ring. For the same reason the appendage in the C2–C3 series may more closely, but not precisely, mimic the binding mode of the more active SS based ligands, and possibly even transfer over to tropane based compounds (cf. Brasofensine).
To better understand the difference between the C1 and the C2–C3 series, the compounds were energy minimized and flexibly superimposed on WIN-35,428. The resulting overlay shows that only the C2–C3 ligands are able to adopt a conformation in which the polar group of the 3α-substituent occupies the position proximal to that of the 2β-polar group in WIN35428.
DAT Arylpiperidine CoMFA Study
(Hongbin Yuan, et al. 2004)A generally recognized pharmacophore model for cocaine and phenyltropane
Phenyltropane
Phenyltropanes were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, they are not the only researchers that have prepared these...
s comprises two electrostatic interactions of the basic nitrogen and the ester group of the C-2 substituent, and one hydrophobic interaction of the C-3 aryl group. This model has been disputed because of the finding that in certain compounds neither the basic N nor the ester group was necessary for high binding affinity and inhibition of MAR. Instead, a hydrophobic pocket was proposed to exist in the vicinity of the C-2 carbon. Carroll et al., however, provided further evidence for an electrostatic interaction at the C-2β-position in a later study.
Other models proposed for the DAT binding site include a linear fashion binding pocket for the 3β-substituted phenyltropane analogs, and a prohibited conical region about 5.5–10Å distant from the 3α-substituted piperidine ring. Noticeably, high potency at the DAT of dimeric piperidine-based esters and amides suggested that the flexible linker combining the two piperidine units was able to adjust its orientation and to avoid unfavorable interactions with the binding site. All these lines of evidence suggest that the DAT binding site is much more complicated than the proposed pharmacophore models.
In an attempt to uncover the details of the DAT binding site, a number of 3D-QSAR studies were performed. Several QSAR/CoMFA studies focused on phenyltropanes concluded that an increased negative electrostatic potential in the regions around the 3β-substituent of the tropane ring and the para-position of the phenyl ring favored high potency in inhibiting the MATs. Wright et al. studied the role of the 3β-substituent of tropanes in binding to the DAT and blocking DA reuptake. Their CoMFA model indicated that the 3β-substituent binding site is barrel-shaped and hydrophobic interactions make a dominant contribution to the binding, which is consistent with the studies of 3α-substituted tropane analogs reported by Newman et al. Newman and co-authors also studied N-substituted tropanes and concluded that the steric interaction of the N-substituent with the DAT is a principle factor for the binding affinity.
Nocaine: Sulfur Appendage
Compound 16eJZ-IV-10
JZ-IV-10 is a piperidine derivative related to nocaine which is a highly potent triple reuptake inhibitor . The eugeroic modafinil, was used as a lead to fuel these compounds' discovery . Although it turns out that the QSAR of the pharmacophoric elements do not appear to be strongly similar...
Patents
- Kowski:
- Ward Neil:
See also:
See also
- List of cocaine analogues
- N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
- JZ-IV-10JZ-IV-10JZ-IV-10 is a piperidine derivative related to nocaine which is a highly potent triple reuptake inhibitor . The eugeroic modafinil, was used as a lead to fuel these compounds' discovery . Although it turns out that the QSAR of the pharmacophoric elements do not appear to be strongly similar...
and other modafinil hybrids - 4-Fluoromeperidine4-Fluoromeperidine4-Fluoropethidine is a drug that is a derivative of pethidine , which combines pethidine's opioid analgesic effects with increased monoamine reuptake inhibition...
and other meperidine analogues. - NET selective.
- Computer.

