
Nernst heat theorem
Encyclopedia

Walther Nernst
Walther Hermann Nernst FRS was a German physical chemist and physicist who is known for his theories behind the calculation of chemical affinity as embodied in the third law of thermodynamics, for which he won the 1920 Nobel Prize in chemistry...
early in the twentieth century and was used in the development of the third law of thermodynamics
Third law of thermodynamics
The third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
.
The theorem
The Nernst heat theorem says that as absolute zero is approached, the entropy change ΔS for a chemical or physical transformation approaches 0. This can be expressed mathematically as follow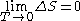
The above equation is a modern statement of the theorem. Nernst often used a form that avoided the concept of entropy.
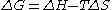 .
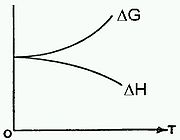
.In the limit of T = 0 the equation reduces to just ΔG = ΔH, as illustrated in the figure shown here, which is supported by experimental data. However, it is known from thermodynamics
Gibbs-Helmholtz equation
The Gibbs–Helmholtz equation is a thermodynamic equation useful for calculating changes in the Gibbs energy of a system as a function of temperature...
that the slope of the ΔG curve is -ΔS. Since the slope shown here reaches the horizontal limit of 0 as T → 0 then the implication is that ΔS → 0, which is the Nernst heat theorem.
The significance of the Nernst heat theorem is that it was later used by Max Planck
Max Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
to give the third law of thermodynamics
Third law of thermodynamics
The third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
, which is that the entropy of all pure, perfectly crystalline homogeneous materials is 0 at absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
.

