_oxide.gif)
Molybdenum(VI) oxide
Encyclopedia
Molybdenum trioxide is chemical compound
with the formula
MoO3. This compound is produced on the largest scale of any molybdenum
compound. It occurs as the rare mineral
molybdite. Its chief application is as an oxidation catalyst and as a raw material for the production of molybdenum metal.
The oxidation state
of molybdenum in this compound is +6.
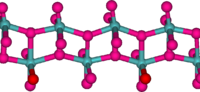 In the gas phase, three oxygen atoms are double bonded to the central molybdenum atom. In the solid state, anhydrous
In the gas phase, three oxygen atoms are double bonded to the central molybdenum atom. In the solid state, anhydrous
MoO3 is composed of layers of distorted MoO6 octahedra in an orthorhombic crystal. The octahedra share edges and form chains which are cross-linked by oxygen atoms to form layers. The octahedra have one short molydenum-oxygen bond to a non-bridging oxygen.
, the chief ore of molybdenum:
The laboratory synthesis entails the acidification of aqueous solutions of sodium molybdate
with perchloric acid
:
The dihydrate loses water readily to give the monohydrate. Both are bright yellow in color.
Molybdenum trioxide dissolves slightly in water to give "molybdic acid
." In base, it dissolves to afford the molybdate anion.
It is also a component of the co-catalyst used in the industrial production of acrylonitrile
by the oxidation of propene and ammonia
.
Because of its layered structure and the ease of the Mo(VI)/Mo(V) couple, MoO3 is of interest in electrochemical devices and displays.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
MoO3. This compound is produced on the largest scale of any molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
compound. It occurs as the rare mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
molybdite. Its chief application is as an oxidation catalyst and as a raw material for the production of molybdenum metal.
The oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of molybdenum in this compound is +6.
Structure

Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
MoO3 is composed of layers of distorted MoO6 octahedra in an orthorhombic crystal. The octahedra share edges and form chains which are cross-linked by oxygen atoms to form layers. The octahedra have one short molydenum-oxygen bond to a non-bridging oxygen.
Preparation and principal reactions
MoO3 is produced industrially by roasting molybdenum disulfideMolybdenum disulfide
Molybdenum disulfide is the inorganic compound with the formula MoS2. This black crystalline sulfide of molybdenum occurs as the mineral molybdenite. It is the principal ore from which molybdenum metal is extracted. The natural amorphous form is known as the rarer mineral jordisite. MoS2 is less...
, the chief ore of molybdenum:
- 2 MoS2 + 7 O2 → 2 MoO3 + 4 SO2
The laboratory synthesis entails the acidification of aqueous solutions of sodium molybdate
Sodium molybdate
Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. It is often found as the dihydrate, Na2MoO4·2H2O.The molybdate anion is tetrahedral. Two sodium cations coordinate with every one anion.-History:...
with perchloric acid
Perchloric acid
Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,...
:
- Na2MoO4 + H2O + 2 HClO4 → MoO3(H2O)2 + 2 NaClO4
The dihydrate loses water readily to give the monohydrate. Both are bright yellow in color.
Molybdenum trioxide dissolves slightly in water to give "molybdic acid
Molybdic acid
Molybdic acid refrs to solid, hydrated forms of molybdenum trioxide and species in aqueous solution.The simplest solid form, the monohydrate, is MoO3·H2O, though the dihydrate is also known. The solid state structure of MoO3·H2O consists of layers of octahedrally coordinated MoO5· units where 4...
." In base, it dissolves to afford the molybdate anion.
Uses
Molybdenum trioxide is used to manufacture molybdenum metal, which serves as an additive to steel and corrosion-resistant alloys. The relevant conversion entails treatment of MoO3 with hydrogen at elevated temperatures:- MoO3 + 3 H2 → Mo + 3 H2O
It is also a component of the co-catalyst used in the industrial production of acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
by the oxidation of propene and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
.
Because of its layered structure and the ease of the Mo(VI)/Mo(V) couple, MoO3 is of interest in electrochemical devices and displays.

