
Mass fraction (chemistry)
Encyclopedia
In chemistry
, the mass fraction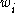 is the fraction of one substance with mass
is the fraction of one substance with mass 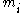 to the mass of the total mixture
to the mass of the total mixture 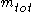 , defined as :
, defined as :
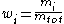
The sum of all the mass fractions is equal to 1:

It is one way of expressing the composition of a mixture in a dimensionless size (mole fraction is another).
For elemental analysis
, mass fraction (or "mass percent composition") can also refer to the fraction of the mass of one element to the total mass of a compound. It can be calculated for any compound using its empirical formula
or its chemical formula
.
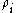 (partial density of that component) to the density
(partial density of that component) to the density
of solution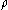 :
:
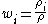
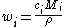
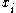 can be calculated using the formula
can be calculated using the formula

where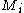 is the molar mass of the component
is the molar mass of the component  and
and 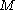 is the average molar mass
is the average molar mass
of the mixture.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the mass fraction
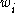 is the fraction of one substance with mass
is the fraction of one substance with mass 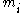 to the mass of the total mixture
to the mass of the total mixture 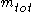 , defined as :
, defined as :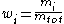
The sum of all the mass fractions is equal to 1:

It is one way of expressing the composition of a mixture in a dimensionless size (mole fraction is another).
For elemental analysis
Elemental analysis
Percent Composition is a process where a sample of some material is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative , and it can be quantitative...
, mass fraction (or "mass percent composition") can also refer to the fraction of the mass of one element to the total mass of a compound. It can be calculated for any compound using its empirical formula
Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic...
or its chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
.
Mass concentration
The mass fraction of a component in a solution is the ratio of the mass concentration of that component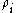 (partial density of that component) to the density
(partial density of that component) to the densityDensity
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of solution
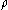 :
: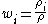
Molar concentration
The relation to molar concentration is like that from above substituting the relation between mass and molar concentration.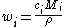
Mass percentage
Multiplying mass fraction by 100 gives the mass percentage, sometimes referred to by the obsolete term weight-weight percentage or weight percent (abbreviated as w/w% or wt%).Mole fraction
The mole fraction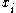 can be calculated using the formula
can be calculated using the formula
where
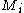 is the molar mass of the component
is the molar mass of the component  and
and 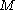 is the average molar mass
is the average molar massMolar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
of the mixture.

