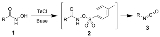
Lossen rearrangement
Overview
Hydroxamic acid
A hydroxamic acid is a class of chemical compounds sharing the same functional group in which an hydroxylamine is inserted into a carboxylic acid. Its general structure is R-CO-NH-OH, with an R as an organic residue, a CO as a carbonyl group, and a hydroxylamine as NH2-OH. They are used as metal...
(1) to an isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
(3) via the formation of an O-acyl, sulfonyl, or phosphoryl intermediate hydroxamic acid O-derivative (2) and then conversion to its conjugate base. Here, 4-Toluenesulfonyl chloride
4-Toluenesulfonyl chloride
4-Toluenesulfonyl chloride is an organic compound with the formula CH3C6H4SO2Cl. This colourless, malodorous solid is a reagent widely used in organic synthesis...
is used to form a sulfonyl O-derivative of hydroxamic acid.
The isocyanate can be used further to generate ureas in the presence of amines (4) or generate amines in the presence of H2O (5).
The mechanism below begins with an O-acylated hydroxamic acid derivative that is treated with base to form an isocyanate that generates an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
and CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
gas in the presence of H2O.
Unanswered Questions

