
Levorotation and dextrorotation
Encyclopedia
Dextrorotation and levorotation (also spelled laevorotation) refer, respectively, to the properties of rotating plane polarized light clockwise (for dextrorotation) or counterclockwise (for levorotation), seen by an observer whom the light is approaching. A compound with dextrorotation is called dextrorotatory or dextrorotary, while a compound with levorotation is called levorotatory or levorotary.
Compounds with these properties are said to have optical activity and consist of chiral
molecules. If a chiral molecule is dextrorotary, its enantiomer
will be levorotary, and vice-versa. In fact, the enantiomers will rotate polarized light the same number of degrees, but in opposite directions.
It is not possible to determine whether a given chiral molecule will be levorotatory or dextrorotatory directly from its configuration, except via detailed computer modeling. In particular, both "R" and "S" stereocenters have the ability to be dextrorotatory or laevorotatory.
.
, not a whole molecule. A molecule with just one stereocenter can be labeled R or S, but a molecule with multiple stereocenters needs more than one label, for example (2R,3S)
.
If there is a pair of enantiomers, each with one stereocenter, then one enantiomer is R and the other is S, and likewise one enantiomer is levorotary and the other is dextrorotary. However, there is no general correlation between these two labels. In some cases the R enantiomer is the dextrorotary enantiomer, and in other cases the R enantiomer is the levorotary enantiomer. The relationship can only be determined on a case-by-case basis with detailed computer modeling or experimental measurements.
[α]. Dextrorotary compounds have a positive specific rotation, while levorotary compounds have negative. Two enantiomers have equal and opposite specific rotations.
The formula for specific rotation is:
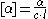
where:
[α] = specific rotation
α = observed rotation
c = concentration of the solution of an enantiomer
l = length of the tube (Polarimeter
tube) in decimeters
The degree of rotation of plane-polarized light depends on the number of chiral molecules that it encounters on its way through the tube of polarimeter (thus, the length of the tube and concentration of the enantiomer). In many cases, it also depends on the temperature and the wavelength of light that is employed.
Compounds with these properties are said to have optical activity and consist of chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
molecules. If a chiral molecule is dextrorotary, its enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
will be levorotary, and vice-versa. In fact, the enantiomers will rotate polarized light the same number of degrees, but in opposite directions.
It is not possible to determine whether a given chiral molecule will be levorotatory or dextrorotatory directly from its configuration, except via detailed computer modeling. In particular, both "R" and "S" stereocenters have the ability to be dextrorotatory or laevorotatory.
The prefixes "(+)-", "(–)-", "d-", "l-", "D-", and "L-"
A dextrorotary compound is often prefixed "(+)-" or "d-". Likewise, a levorotary compound is often prefixed "(–)-" or "l-". These "d-" and "l-" prefixes should not be confused with the "D-" and "L-" prefixes based on the actual configuration of each enantiomer, with the version synthesized from naturally occurring (+)-glyceraldehyde being considered the D- form. For example, nine of the nineteen L-amino acids commonly found in proteins are dextrorotatory (at a wavelength of 589 nm), and D-fructose is also referred to as levulose because it is levorotatory. See the article: Chirality (chemistry)Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
.
The prefixes "(R)-" and "(S)-"
The R and S prefixes are different from the preceding ones in that the labels R and S characterize a specific stereocenterStereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
, not a whole molecule. A molecule with just one stereocenter can be labeled R or S, but a molecule with multiple stereocenters needs more than one label, for example (2R,3S)
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
.
If there is a pair of enantiomers, each with one stereocenter, then one enantiomer is R and the other is S, and likewise one enantiomer is levorotary and the other is dextrorotary. However, there is no general correlation between these two labels. In some cases the R enantiomer is the dextrorotary enantiomer, and in other cases the R enantiomer is the levorotary enantiomer. The relationship can only be determined on a case-by-case basis with detailed computer modeling or experimental measurements.
Specific rotation
A standard measure of the degree to which a compound is dextrorotary or levorotary is the quantity called the specific rotationSpecific rotation
In stereochemistry, the specific rotation of a chemical compound [α] is defined as the observed angle of optical rotation α when plane-polarized light is passed through a sample with a path length of 1 decimeter and a sample concentration of 1 gram per 1 millilitre. It is the main property used to...
[α]. Dextrorotary compounds have a positive specific rotation, while levorotary compounds have negative. Two enantiomers have equal and opposite specific rotations.
The formula for specific rotation is:
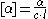
where:
[α] = specific rotation
α = observed rotation
c = concentration of the solution of an enantiomer
l = length of the tube (Polarimeter
Polarimeter
A polarimeter is a scientific instrument used to measure the angle of rotation caused by passing polarized light through an optically active substance....
tube) in decimeters
The degree of rotation of plane-polarized light depends on the number of chiral molecules that it encounters on its way through the tube of polarimeter (thus, the length of the tube and concentration of the enantiomer). In many cases, it also depends on the temperature and the wavelength of light that is employed.

