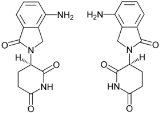
Lenalidomide
Encyclopedia
Lenalidomide initially known as CC-5013 and marketed as Revlimid by Celgene
, is a derivative
of thalidomide
introduced in 2004.
It was initially intended as a treatment for multiple myeloma
, for which thalidomide is an accepted therapeutic treatment. Lenalidomide has also shown efficacy in the class of hematological disorders known as myelodysplastic syndrome
s (MDS). Lenalidomide and bortezomib
are considered therapeutic breakthroughs in the treatment of myeloma, which generally carries a poor prognosis.
, and they can be simplified by organizing them as mechanisms of action in vitro and in vivo. In vitro, lenalidomide has three main activities: direct anti-tumor effect, inhibition of the microenvironment support for tumor cells, and immunomodulatory role. In vivo, lenalidomide induces tumor cell apoptosis
directly and indirectly by inhibition of bone marrow stromal cell
support, by anti-angiogenic and anti-osteoclastogenic
effects, and by immunomodulatory activity. Lenalidomide has a broad range of activities that can be exploited to treat many hematologic and solid cancers.
. It is a small molecular analog of thalidomide that was originally found based on its ability to effectively inhibit tumor necrosis factor production. Lenalidomide is 50,000 times more potent than thalidomide in inhibiting tumor necrosis factor-alpha, and has less severe adverse drug reactions. In a phase III clinical study, Weber et al. found that lenalidomide plus dexamethasone
in patients with relapsed or refractory multiple myeloma was superior to the old treatment of multiple myeloma consisting of high-dose dexamethasone alone.
Nonetheless, lenalidomide, like its parent compound thalidomide, may cause venous thromboembolism (VTE), a potentially serious complication with their use. Bennett et al. have reviewed incidents of lenalidomide-associated VTE among patients with multiple myeloma. They have found that there are high rates of VTE when patients with multiple myeloma received thalidomide or lenalidomide in conjunction with dexamethasone
, melphalan
, or doxorubicin
. When lenalidomide and dexamethasone are used to treat multiple myeloma, a median of 14% of patients had VTE (range,3-75%). Patients who took prophylaxis to treat lenalidomide-associated VTE, such as aspirin, thromboembolism rates were found to be lower than without prophylaxis, frequently lower than 10%. Clearly, thromboembolism is a serious adverse drug reaction associated with lenalidomide, as well as thalidomide. In fact, a black box warning is included in the package insert for lenalidomide, indicating that lenalidomide-dexamethasone treatment for multiple myeloma is complicated by high rates of thromboembolism.
Currently, clinical trials are underway to further test the efficacy of lenalidomide to treat multiple myeloma and how to prevent the lenalidomide associated venous thromboembolism.
(NICE) issued a Final Appraisal Determination (FAD) approving lenalidomide, in combination with dexamethasone, as an option to treat patients who suffer from multiple myeloma who have received two or more prior therapies in England and Wales.
It was approved by the FDA on December 27, 2005 for patients with low or intermediate-1 risk MDS with 5q- with or without additional cytogenetic abnormalities. A completed Phase II, multi-centre, single-arm, open-label study evaluated the efficacy and safety of Revlimid monotherapy treatment for achieving haematopoietic improvement in red blood cell (RBC) transfusion dependent subjects with low- or intermediate-1-risk MDS associated with a deletion 5q cytogenetic abnormality.
63.8% of subjects had achieved RBC-transfusion independence accompanied by a median increase of 5.8 g/dL in blood Hgb concentration from baseline to the maximum value during the response period. Major cytogenetic responses were observed in 44.2% and minor cytogenetic responses were observed in 24.2% of the evaluable subjects. Improvements in bone marrow morphology were also observed. The results of this study demonstrate the efficacy of Revlimid for the treatment of subjects with Low- or Intermediate-1-risk MDS and an associated del 5 cytogenetic abnormality.
, as well as non-Hodgkin's Lymphoma, Chronic Lymphocytic Leukemia
and solid tumor cancers, such as carcinoma
of the pancreas
.
which is known to be teratogenic. While laboratory tests have suggested lenalidomide is not teratogenic it is categorized as such because of its structural similarities with thalidomide. It therefore has the pregnancy category
X and cannot be prescribed for women who are pregnant or who might be conceiving. For this reason, the drug is only available in the United States
(under the name Revlimid) through a restricted distribution system called RevAssistSM.
Other potential side effects are thrombosis
, pulmonary embolus, and hepatotoxicity
, as well as bone marrow toxicity resulting in neutropenia
and thrombocytopenia
. Myelosuppression is the major dose-limiting toxicity, which is contrary to experience with thalidomide.
In March 2008, the U.S. Food and Drug Administration (FDA) included lenalidomide on a list of 20 prescription drugs under investigation for potential safety problems. The drug is being investigated for possibly increasing the risk of developing Stevens–Johnson syndrome, a life-threatening condition affecting the skin.
Celgene
Celgene Corporation is a manufacturer of drug therapies for cancer and inflammatory disorders. It is incorporated in Delaware and headquartered in Summit, New Jersey...
, is a derivative
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
of thalidomide
Thalidomide
Thalidomide was introduced as a sedative drug in the late 1950s that was typically used to cure morning sickness. In 1961, it was withdrawn due to teratogenicity and neuropathy. There is now a growing clinical interest in thalidomide, and it is introduced as an immunomodulatory agent used...
introduced in 2004.
It was initially intended as a treatment for multiple myeloma
Multiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
, for which thalidomide is an accepted therapeutic treatment. Lenalidomide has also shown efficacy in the class of hematological disorders known as myelodysplastic syndrome
Myelodysplastic syndrome
The myelodysplastic syndromes are a diverse collection of hematological medical conditions that involve ineffective production of the myeloid class of blood cells....
s (MDS). Lenalidomide and bortezomib
Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma...
are considered therapeutic breakthroughs in the treatment of myeloma, which generally carries a poor prognosis.
Mechanism of action
Lenalidomide has been used to successfully treat both inflammatory disorders and cancers in the past 10 years. There are multiple mechanisms of actionMechanism of action
In pharmacology, the term mechanism of action refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect...
, and they can be simplified by organizing them as mechanisms of action in vitro and in vivo. In vitro, lenalidomide has three main activities: direct anti-tumor effect, inhibition of the microenvironment support for tumor cells, and immunomodulatory role. In vivo, lenalidomide induces tumor cell apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
directly and indirectly by inhibition of bone marrow stromal cell
Stromal cell
In cell biology, stromal cells are connective tissue cells of any organ, for example in the uterine mucosa , prostate, bone marrow, and the ovary. They are cells that support the function of the parenchymal cells of that organ...
support, by anti-angiogenic and anti-osteoclastogenic
Osteoclast
An osteoclast is a type of bone cell that removes bone tissue by removing its mineralized matrix and breaking up the organic bone . This process is known as bone resorption. Osteoclasts were discovered by Kolliker in 1873...
effects, and by immunomodulatory activity. Lenalidomide has a broad range of activities that can be exploited to treat many hematologic and solid cancers.
Treatment of multiple myeloma
Multiple myeloma is a rare cancer of the blood, characterized by accumulation of a plasma cell clone in the bone marrow. Lenalidomide is one of the novel drug agents used to treat multiple myelomaMultiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
. It is a small molecular analog of thalidomide that was originally found based on its ability to effectively inhibit tumor necrosis factor production. Lenalidomide is 50,000 times more potent than thalidomide in inhibiting tumor necrosis factor-alpha, and has less severe adverse drug reactions. In a phase III clinical study, Weber et al. found that lenalidomide plus dexamethasone
Dexamethasone
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs. It acts as an anti-inflammatory and immunosuppressant...
in patients with relapsed or refractory multiple myeloma was superior to the old treatment of multiple myeloma consisting of high-dose dexamethasone alone.
Nonetheless, lenalidomide, like its parent compound thalidomide, may cause venous thromboembolism (VTE), a potentially serious complication with their use. Bennett et al. have reviewed incidents of lenalidomide-associated VTE among patients with multiple myeloma. They have found that there are high rates of VTE when patients with multiple myeloma received thalidomide or lenalidomide in conjunction with dexamethasone
Dexamethasone
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs. It acts as an anti-inflammatory and immunosuppressant...
, melphalan
Melphalan
Melphalan hydrochloride is a chemotherapy drug belonging to the class of nitrogen mustard alkylating agents.An alkylating agent adds an alkyl group to DNA...
, or doxorubicin
Doxorubicin
Doxorubicin INN is a drug used in cancer chemotherapy. It is an anthracycline antibiotic, closely related to the natural product daunomycin, and like all anthracyclines, it works by intercalating DNA....
. When lenalidomide and dexamethasone are used to treat multiple myeloma, a median of 14% of patients had VTE (range,3-75%). Patients who took prophylaxis to treat lenalidomide-associated VTE, such as aspirin, thromboembolism rates were found to be lower than without prophylaxis, frequently lower than 10%. Clearly, thromboembolism is a serious adverse drug reaction associated with lenalidomide, as well as thalidomide. In fact, a black box warning is included in the package insert for lenalidomide, indicating that lenalidomide-dexamethasone treatment for multiple myeloma is complicated by high rates of thromboembolism.
Currently, clinical trials are underway to further test the efficacy of lenalidomide to treat multiple myeloma and how to prevent the lenalidomide associated venous thromboembolism.
Use in USA
On June 29, 2006, lenalidomide received U.S. Food and Drug Administration (FDA) clearance for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy.Use in the UK
On 23 April 2009, The National Institute for Health and Clinical ExcellenceNational Institute for Health and Clinical Excellence
The National Institute for Health and Clinical Excellence is a special health authority of the English National Health Service , serving both English NHS and the Welsh NHS...
(NICE) issued a Final Appraisal Determination (FAD) approving lenalidomide, in combination with dexamethasone, as an option to treat patients who suffer from multiple myeloma who have received two or more prior therapies in England and Wales.
Treatment of myelodysplastic syndromes
With myelodysplastic syndromes (MDS), the best results of lenalidomide were obtained in patients with deletion 5q.It was approved by the FDA on December 27, 2005 for patients with low or intermediate-1 risk MDS with 5q- with or without additional cytogenetic abnormalities. A completed Phase II, multi-centre, single-arm, open-label study evaluated the efficacy and safety of Revlimid monotherapy treatment for achieving haematopoietic improvement in red blood cell (RBC) transfusion dependent subjects with low- or intermediate-1-risk MDS associated with a deletion 5q cytogenetic abnormality.
63.8% of subjects had achieved RBC-transfusion independence accompanied by a median increase of 5.8 g/dL in blood Hgb concentration from baseline to the maximum value during the response period. Major cytogenetic responses were observed in 44.2% and minor cytogenetic responses were observed in 24.2% of the evaluable subjects. Improvements in bone marrow morphology were also observed. The results of this study demonstrate the efficacy of Revlimid for the treatment of subjects with Low- or Intermediate-1-risk MDS and an associated del 5 cytogenetic abnormality.
Treatment of other cancers
Lenalidomide is undergoing clinical trial as a treatment for Hodgkin's LymphomaHodgkin's lymphoma
Hodgkin's lymphoma, previously known as Hodgkin's disease, is a type of lymphoma, which is a cancer originating from white blood cells called lymphocytes...
, as well as non-Hodgkin's Lymphoma, Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia
B-cell chronic lymphocytic leukemia , also known as chronic lymphoid leukemia , is the most common type of leukemia. Leukemias are cancers of the white blood cells . CLL affects B cell lymphocytes. B cells originate in the bone marrow, develop in the lymph nodes, and normally fight infection by...
and solid tumor cancers, such as carcinoma
Carcinoma
Carcinoma is the medical term for the most common type of cancer occurring in humans. Put simply, a carcinoma is a cancer that begins in a tissue that lines the inner or outer surfaces of the body, and that generally arises from cells originating in the endodermal or ectodermal germ layer during...
of the pancreas
Pancreas
The pancreas is a gland organ in the digestive and endocrine system of vertebrates. It is both an endocrine gland producing several important hormones, including insulin, glucagon, and somatostatin, as well as a digestive organ, secreting pancreatic juice containing digestive enzymes that assist...
.
Risks
Lenalidomide is related to thalidomideThalidomide
Thalidomide was introduced as a sedative drug in the late 1950s that was typically used to cure morning sickness. In 1961, it was withdrawn due to teratogenicity and neuropathy. There is now a growing clinical interest in thalidomide, and it is introduced as an immunomodulatory agent used...
which is known to be teratogenic. While laboratory tests have suggested lenalidomide is not teratogenic it is categorized as such because of its structural similarities with thalidomide. It therefore has the pregnancy category
Pregnancy category
The pregnancy category of a pharmaceutical agent is an assessment of the risk of fetal injury due to the pharmaceutical, if it is used as directed by the mother during pregnancy. It does not include any risks conferred by pharmaceutical agents or their metabolites that are present in breast...
X and cannot be prescribed for women who are pregnant or who might be conceiving. For this reason, the drug is only available in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
(under the name Revlimid) through a restricted distribution system called RevAssistSM.
Other potential side effects are thrombosis
Thrombosis
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets and fibrin to form a blood clot to prevent blood loss...
, pulmonary embolus, and hepatotoxicity
Hepatotoxicity
Hepatotoxicity implies chemical-driven liver damage.The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic ranges, may injure...
, as well as bone marrow toxicity resulting in neutropenia
Neutropenia
Neutropenia, from Latin prefix neutro- and Greek suffix -πενία , is a granulocyte disorder characterized by an abnormally low number of neutrophils, the most important type of white blood cell...
and thrombocytopenia
Thrombocytopenia
Thrombocytopenia is a relative decrease of platelets in blood.A normal human platelet count ranges from 150,000 to 450,000 platelets per microliter of blood. These limits are determined by the 2.5th lower and upper percentile, so values outside this range do not necessarily indicate disease...
. Myelosuppression is the major dose-limiting toxicity, which is contrary to experience with thalidomide.
In March 2008, the U.S. Food and Drug Administration (FDA) included lenalidomide on a list of 20 prescription drugs under investigation for potential safety problems. The drug is being investigated for possibly increasing the risk of developing Stevens–Johnson syndrome, a life-threatening condition affecting the skin.
External links
- Official website Includes list of adverse reactions
- Prescribing Information
- International Myeloma Foundation article on Revlimid
- multiplemyeloma.org Revlimid April 2007 Summary
- Medical News Phase II trial results Dec 2004

