
Laplace pressure
Encyclopedia
The Laplace pressure is the pressure
difference between the inside and the outside of a bubble
or droplet. The effect is caused by the surface tension
of the interface between liquid and gas.
The Laplace pressure is given as
where in the case of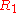 being equal to
being equal to 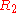 ,
, 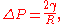
where
This relation can be readily derived from the Young–Laplace equation
.
A common example of use is finding the pressure inside an air bubble in pure water, where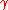 = 72 mN/m. The extra pressure inside the bubble is given here for three bubble sizes:
= 72 mN/m. The extra pressure inside the bubble is given here for three bubble sizes:
A 1 mm bubble has negligible extra pressure. Only when the diameter is ~ 3 microns, the bubble has an extra atmosphere inside than outside. When the bubble is only several hundred nanometers, the pressure inside can be several atmospheres. One should bear in mind that the surface tension in the numerator can be much smaller in the presence of surfactants or contaminants. The same calculation can be done for small oil droplets in water, where even in the presence of surfactants and a fairly low interfacial tension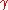 = 5–10 mN/m, the pressure inside 100 nm diameter droplets can reach several atmospheres. Such nanoemulsions
= 5–10 mN/m, the pressure inside 100 nm diameter droplets can reach several atmospheres. Such nanoemulsions
can be antibacterial because the large pressure inside the oil droplets can cause them to attach to bacteria, and simply merge with them, swell them, and "pop" them.
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
difference between the inside and the outside of a bubble
Liquid bubble
A bubble is a globule of one substance in another, usually gas in a liquid.Due to the Marangoni effect, bubbles may remain intact when they reach the surface of the immersive substance.-Common examples:...
or droplet. The effect is caused by the surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
of the interface between liquid and gas.
The Laplace pressure is given as
where in the case of
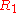 being equal to
being equal to 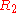 ,
, 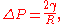
where
- Pinside is the pressure inside the bubble or droplet
- Poutside is the pressure outside the bubble or droplet
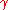 (also denoted as
(also denoted as  ) is the surface tension
) is the surface tension- R is the radius of the bubble
This relation can be readily derived from the Young–Laplace equation
Young–Laplace equation
In physics, the Young–Laplace equation is a nonlinear partial differential equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or wall tension, although usage on the...
.
A common example of use is finding the pressure inside an air bubble in pure water, where
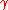 = 72 mN/m. The extra pressure inside the bubble is given here for three bubble sizes:
= 72 mN/m. The extra pressure inside the bubble is given here for three bubble sizes:
| Bubble diameter (2r) (microns) |  (Pa) (Pa) |
 (atm) (atm) |
|---|---|---|
| 1000 | 288 | 0.00284 |
| 3.0 | 96000 | 0.947 |
| 0.3 | 960000 | 9.474 |
A 1 mm bubble has negligible extra pressure. Only when the diameter is ~ 3 microns, the bubble has an extra atmosphere inside than outside. When the bubble is only several hundred nanometers, the pressure inside can be several atmospheres. One should bear in mind that the surface tension in the numerator can be much smaller in the presence of surfactants or contaminants. The same calculation can be done for small oil droplets in water, where even in the presence of surfactants and a fairly low interfacial tension
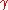 = 5–10 mN/m, the pressure inside 100 nm diameter droplets can reach several atmospheres. Such nanoemulsions
= 5–10 mN/m, the pressure inside 100 nm diameter droplets can reach several atmospheres. Such nanoemulsionsEmulsion
An emulsion is a mixture of two or more liquids that are normally immiscible . Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion is used when both the dispersed and the...
can be antibacterial because the large pressure inside the oil droplets can cause them to attach to bacteria, and simply merge with them, swell them, and "pop" them.


