
K-alpha
Encyclopedia
X-ray spectroscopy
X-ray spectroscopy is a gathering name for several spectroscopic techniques for characterization of materials by using x-ray excitation.-Characteristic X-ray Spectroscopy:...
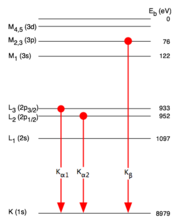
, K-alpha emission lines result when an electron transitions to the innermost "K" shell (principal quantum number 1) from a 2p orbital of the second or "L" shell (with principal quantum number 2). The line is actually a doublet, with slightly different energies depending on spin-orbit interaction
Spin-orbit interaction
In quantum physics, the spin-orbit interaction is any interaction of a particle's spin with its motion. The first and best known example of this is that spin-orbit interaction causes shifts in an electron's atomic energy levels due to electromagnetic interaction between the electron's spin and...
energy between the electron spin and the orbital momentum of the 2p orbital. K-alpha is typically by far the strongest X-ray spectral line for an element bombarded with energy sufficient to cause maximally intense X-ray emission.
The analogous K-alpha spectra line in hydrogen is known as Lyman alpha; however because of hydrogen's small nuclear charge, this line is in the ultraviolet, not the X-ray range. See Siegbahn notation
Siegbahn notation
The Siegbahn notation is used in X-ray spectroscopy to name the spectral lines that are characteristic to elements. It was created by Manne Siegbahn....
for the newer IUPAC-recommended spectral notation system.
An example of K-alpha lines are those seen for iron as iron atoms radiating X-rays spiralling into a black hole
Black hole
A black hole is a region of spacetime from which nothing, not even light, can escape. The theory of general relativity predicts that a sufficiently compact mass will deform spacetime to form a black hole. Around a black hole there is a mathematically defined surface called an event horizon that...
at the center of a galaxy http://arxiv.org/abs/astro-ph/0405337. For such purposes, the frequency of the line is adequately calculated to 2-digit accuracy by the use of Moseley's law
Moseley's law
Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913...
. This formula is 10.2 eV multiplied by the square of a quantity one less than the atomic number of the element in question (atomic number minus 1). For example, K-alpha for iron (Z = 26) is calculated in this fashion as 10.2 eV (25)2 = 6.38 keV. For astrophysical purposes, Doppler
Doppler effect
The Doppler effect , named after Austrian physicist Christian Doppler who proposed it in 1842 in Prague, is the change in frequency of a wave for an observer moving relative to the source of the wave. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from...
and other effects (such as gravitational broadening) show the iron line to no better accuracy than 6.4 keV. http://www.journals.uchicago.edu/cgi-bin/resolve?id=doi:10.1086/340992&erFrom=-6703307617883835216Guest
Values of Transition Energies
- Values of different kinds of transition energies like K
 , K
, K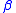 , L
, L , L
, L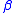 and so on for different elements can be found in the NIST X-Ray Transition Energies Database..
and so on for different elements can be found in the NIST X-Ray Transition Energies Database.. - K-alpha emission values for hydrogen-like and helium-like ions can be found on Table 1-5 of the LNBL X-Ray Data Booklet

