
Isotopes of strontium
Encyclopedia
The alkaline earth metal
strontium
(Sr) has four stable, naturally occurring isotope
s:
84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%). It has a standard atomic mass of 87.62(1) u.
Only 87Sr is radiogenic; it is produced by decay from the radioactive alkali metal 87Rb
, which has a half-life
of 4.88 × 1010 years. Thus, there are two sources of 87Sr in any material: that formed during primordial nucleo-synthesis along with 84Sr, 86Sr and 88Sr, as well as that formed by radioactive decay of 87Rb. The ratio 87Sr/86Sr is the parameter typically reported in geologic
investigations; ratios in minerals and rock
s have values ranging from about 0.7 to greater than 4.0. Because strontium has an electron configuration
similar to that of calcium
, it readily substitutes for Ca in mineral
s.
Twenty-nine unstable isotopes are known to exist, the longest-lived of which are 90Sr with a half-life of 28.9 years and 85Sr with a half-life of 64.853 days. Of importance are strontium-89 (89Sr) with a half-life
of 50.57 days, and strontium-90 (90Sr)
. They decay by emitting an electron
and an anti-neutrino (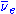 ) in beta decay
) in beta decay
(β− decay) to become yttrium
:


89Sr is an artificial radioisotope which is used in treatment of bone cancer. In circumstances where cancer patients have widespread and painful bony metastases, the administration of 89Sr results in the delivery of beta particles directly to the area of bony problem, where calcium turnover is greatest.
90Sr is a by-product of nuclear fission
which is found in nuclear fallout
and presents a health problem since it substitutes for calcium in bone
, preventing expulsion from the body. Because it is a long-lived high-energy beta emitter, it is used in SNAP (Systems for Nuclear Auxiliary Power) devices. These devices hold promise for use in spacecraft
, remote weather stations, navigational buoys, etc., where a lightweight, long-lived, nuclear-electric power source is required. The 1986 Chernobyl nuclear accident contaminated a vast area with 90Sr.
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
(Sr) has four stable, naturally occurring isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s:
84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%). It has a standard atomic mass of 87.62(1) u.
Only 87Sr is radiogenic; it is produced by decay from the radioactive alkali metal 87Rb
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
, which has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 4.88 × 1010 years. Thus, there are two sources of 87Sr in any material: that formed during primordial nucleo-synthesis along with 84Sr, 86Sr and 88Sr, as well as that formed by radioactive decay of 87Rb. The ratio 87Sr/86Sr is the parameter typically reported in geologic
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
investigations; ratios in minerals and rock
Rock (geology)
In geology, rock or stone is a naturally occurring solid aggregate of minerals and/or mineraloids.The Earth's outer solid layer, the lithosphere, is made of rock. In general rocks are of three types, namely, igneous, sedimentary, and metamorphic...
s have values ranging from about 0.7 to greater than 4.0. Because strontium has an electron configuration
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
similar to that of calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
, it readily substitutes for Ca in mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
s.
Twenty-nine unstable isotopes are known to exist, the longest-lived of which are 90Sr with a half-life of 28.9 years and 85Sr with a half-life of 64.853 days. Of importance are strontium-89 (89Sr) with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 50.57 days, and strontium-90 (90Sr)
Strontium-90
Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard...
. They decay by emitting an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
and an anti-neutrino (
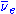 ) in beta decay
) in beta decayBeta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
(β− decay) to become yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
:


89Sr is an artificial radioisotope which is used in treatment of bone cancer. In circumstances where cancer patients have widespread and painful bony metastases, the administration of 89Sr results in the delivery of beta particles directly to the area of bony problem, where calcium turnover is greatest.
90Sr is a by-product of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
which is found in nuclear fallout
Nuclear fallout
Fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and shock wave have passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes...
and presents a health problem since it substitutes for calcium in bone
Bone
Bones are rigid organs that constitute part of the endoskeleton of vertebrates. They support, and protect the various organs of the body, produce red and white blood cells and store minerals. Bone tissue is a type of dense connective tissue...
, preventing expulsion from the body. Because it is a long-lived high-energy beta emitter, it is used in SNAP (Systems for Nuclear Auxiliary Power) devices. These devices hold promise for use in spacecraft
Spacecraft
A spacecraft or spaceship is a craft or machine designed for spaceflight. Spacecraft are used for a variety of purposes, including communications, earth observation, meteorology, navigation, planetary exploration and transportation of humans and cargo....
, remote weather stations, navigational buoys, etc., where a lightweight, long-lived, nuclear-electric power source is required. The 1986 Chernobyl nuclear accident contaminated a vast area with 90Sr.
Table
| nuclide symbol |
Z(p Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... ) |
N(n Neutron The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of... ) |
isotopic mass (u) |
half-life | decay mode(s)Abbreviations: EC: Electron capture Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... IT: Isomeric transition Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... |
daughter isotope(s)Bold for stable isotopes, bold italic for nearly-stable isotopes (half-life longer than the age of the universe Age of the universe The age of the universe is the time elapsed since the Big Bang posited by the most widely accepted scientific model of cosmology. The best current estimate of the age of the universe is 13.75 ± 0.13 billion years within the Lambda-CDM concordance model... ) |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|
| excitation energy | |||||||||
| 73Sr | 38 | 35 | 72.96597(64)# | >25 ms | β+ Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... (>99.9%) |
73Rb | 1/2-# | ||
| β+, p Proton emission Proton emission is a type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state of very... (<.1%) |
72Kr | ||||||||
| 74Sr | 38 | 36 | 73.95631(54)# | 50# ms [>1.5 µs] | β+ | 74Rb | 0+ | ||
| 75Sr | 38 | 37 | 74.94995(24) | 88(3) ms | β+ (93.5%) | 75Rb | (3/2-) | ||
| β+, p (6.5%) | 74Kr | ||||||||
| 76Sr | 38 | 38 | 75.94177(4) | 7.89(7) s | β+ | 76Rb | 0+ | ||
| 77Sr | 38 | 39 | 76.937945(10) | 9.0(2) s | β+ (99.75%) | 77Rb | 5/2+ | ||
| β+, p (.25%) | 76Kr | ||||||||
| 78Sr | 38 | 40 | 77.932180(8) | 159(8) s | β+ | 78Rb | 0+ | ||
| 79Sr | 38 | 41 | 78.929708(9) | 2.25(10) min | β+ | 79Rb | 3/2(-) | ||
| 80Sr | 38 | 42 | 79.924521(7) | 106.3(15) min | β+ | 80Rb | 0+ | ||
| 81Sr | 38 | 43 | 80.923212(7) | 22.3(4) min | β+ | 81Rb | 1/2- | ||
| 82Sr | 38 | 44 | 81.918402(6) | 25.36(3) d | EC Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... |
82Rb | 0+ | ||
| 83Sr | 38 | 45 | 82.917557(11) | 32.41(3) h | β+ | 83Rb | 7/2+ | ||
| 83mSr | 259.15(9) keV | 4.95(12) s | IT | 83Sr | 1/2- | ||||
| 84Sr | 38 | 46 | 83.913425(3) | Observationally StableBelieved to decay by β+β+ to 84Kr | 0+ | 0.0056(1) | 0.0055-0.0058 | ||
| 85Sr | 38 | 47 | 84.912933(3) | 64.853(8) d | EC | 85Rb | 9/2+ | ||
| 85mSr | 238.66(6) keV | 67.63(4) min | IT (86.6%) | 85Sr | 1/2- | ||||
| β+ (13.4%) | 85Rb | ||||||||
| 86Sr | 38 | 48 | 85.9092602(12) | Stable | 0+ | 0.0986(1) | 0.0975-0.0999 | ||
| 86mSr | 2955.68(21) keV | 455(7) ns | 8+ | ||||||
| 87SrUsed in rubidium-strontium dating Rubidium-strontium dating The rubidium-strontium dating method is a radiometric dating technique that geologists use to determine the age of rocks.Development of this process was aided by Fritz Strassmann, who later went on to discover nuclear fission with Otto Hahn and Lise Meitner.... |
38 | 49 | 86.9088771(12) | Stable | 9/2+ | 0.0700(1) | 0.0694-0.0714 | ||
| 87mSr | 388.533(3) keV | 2.815(12) h | IT (99.7%) | 87Sr | 1/2- | ||||
| EC (.3%) | 87Rb | ||||||||
| 88SrFission product Fission product Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The... |
38 | 50 | 87.9056121(12) | Stable | 0+ | 0.8258(1) | 0.8229-0.8275 | ||
| 89Sr | 38 | 51 | 88.9074507(12) | 50.57(3) d | β- | 89Y | 5/2+ | ||
| 90Sr Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... |
38 | 52 | 89.907738(3) | 28.90(3) a | β- | 90Y | 0+ | ||
| 91Sr | 38 | 53 | 90.910203(5) | 9.63(5) h | β- | 91Y | 5/2+ | ||
| 92Sr | 38 | 54 | 91.911038(4) | 2.66(4) h | β- | 92Y | 0+ | ||
| 93Sr | 38 | 55 | 92.914026(8) | 7.423(24) min | β- | 93Y | 5/2+ | ||
| 94Sr | 38 | 56 | 93.915361(8) | 75.3(2) s | β- | 94Y | 0+ | ||
| 95Sr | 38 | 57 | 94.919359(8) | 23.90(14) s | β- | 95Y | 1/2+ | ||
| 96Sr | 38 | 58 | 95.921697(29) | 1.07(1) s | β- | 96Y | 0+ | ||
| 97Sr | 38 | 59 | 96.926153(21) | 429(5) ms | β- (99.95%) | 97Y | 1/2+ | ||
| β-, n Neutron emission Neutron emission is a type of radioactive decay of atoms containing excess neutrons, in which a neutron is simply ejected from the nucleus. Two examples of isotopes which emit neutrons are helium-5 and beryllium-13... (.05%) |
96Y | ||||||||
| 97m1Sr | 308.13(11) keV | 170(10) ns | (7/2)+ | ||||||
| 97m2Sr | 830.8(2) keV | 255(10) ns | (11/2-)# | ||||||
| 98Sr | 38 | 60 | 97.928453(28) | 0.653(2) s | β- (99.75%) | 98Y | 0+ | ||
| β-, n (.25%) | 97Y | ||||||||
| 99Sr | 38 | 61 | 98.93324(9) | 0.269(1) s | β- (99.9%) | 99Y | 3/2+ | ||
| β-, n (.1%) | 98Y | ||||||||
| 100Sr | 38 | 62 | 99.93535(14) | 202(3) ms | β- (99.02%) | 100Y | 0+ | ||
| β-, n (.98%) | 99Y | ||||||||
| 101Sr | 38 | 63 | 100.94052(13) | 118(3) ms | β- (97.63%) | 101Y | (5/2-) | ||
| β-, n (2.37%) | 100Y | ||||||||
| 102Sr | 38 | 64 | 101.94302(12) | 69(6) ms | β- (94.5%) | 102Y | 0+ | ||
| β-, n (5.5%) | 101Y | ||||||||
| 103Sr | 38 | 65 | 102.94895(54)# | 50# ms [>300 ns] | β- | 103Y | |||
| 104Sr | 38 | 66 | 103.95233(75)# | 30# ms [>300 ns] | β- | 104Y | 0+ | ||
| 105Sr | 38 | 67 | 104.95858(75)# | 20# ms [>300 ns] | |||||

