
Infrared gas analyzer
Encyclopedia
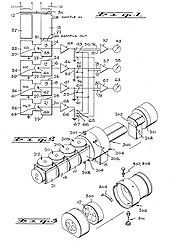
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
of an emitted infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
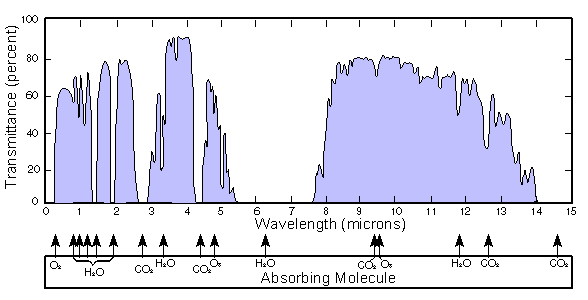
light source through a certain air sample. Trace gases found in the Earth's atmosphere get excited under specific wavelengths found in the infrared range. The concept behind the technology can be understood when considering the greenhouse effect
Greenhouse effect
The greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere...
. When sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
hits the Earth's surface, the incoming short wave radiation gets turned into long wave infrared radiation
Outgoing longwave radiation
Outgoing Longwave Radiation is the energy leaving the earth as infrared radiation at low energy. OLR is a critical component of the Earth’s radiation budget and represents the total radiation going to space emitted by the atmosphere...
that is reflected back into space. If the planet has a thick atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
, much of this radiation is absorbed by the "greenhouse gases" in the atmosphere which act as an insulative
Insulation
Insulation means:* Building insulation, added to buildings for comfort and energy efficiency* Soundproofing, also known as acoustic insulation, any means of reducing the intensity of sound...
blanket. The infrared gas analyzer works using a similar principle.
Infrared gas analyzers usually have two chambers, one is a reference chamber while the other chamber is a measurement chamber. Infrared light is emitted from some type of source on one end of the chamber, passes through a series of chambers that contains given quantities of the various gases in question.
Principals of Operation
The design from 1975 (pictured above) is a Nondispersive infrared sensor. It is the first improved analyser that is able to detect more than one component of a sample gas at one time. Earlier analysers were held back by the fact that a particular gas also has lower absorption bands in the infrared spectrum in addition to its principal absorption band, and either of these bands may overlap the principal absorption band of a second gas.The invention of 1975 has as many detectors as the number of gases to be measured. Each detector has 2 chambers which both have an optically aligned infrared source and detector, and are both filled with one of the gases in the sample of air to be analysed. Lying in the optical path are two cells with transparent ends. One contains a reference gas and one will contain the gas to be analysed. Between the infrared source and the cells is a modulator which interrupts the beams of energy.
The output from each detector is combined with the output from any other detector which is measuring a signal opposite to the principal signal of each detector. The amount of signal from other detectors is the amount that will offset the proportion of the total signal that corresponds to the interference. This interference is from gases with a principal or lower absorption band that is the same as the principal band of the gas being measured.
For instance, if the analyser is to measure carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, the chambers must contain a certain amount of these gases. The infrared light is emitted and passes through the sample gas, a reference gas with a known mixture of the gases in question and then through the "detector" chambers containing the pure forms of the gases in question. When a "detector" chamber absorbs some of the infrared radiation, it heats up and expands. This causes a rise in pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
within the sealed vessel that can be detected either with a pressure transducer
Transducer
A transducer is a device that converts one type of energy to another. Energy types include electrical, mechanical, electromagnetic , chemical, acoustic or thermal energy. While the term transducer commonly implies the use of a sensor/detector, any device which converts energy can be considered a...
or with a similar device. The combination of output voltages from the detector chambers from the sample gas can then be compared to the output voltages from the reference chamber.
The latest Infrared Gas Analyzers
Like earlier infrared gas analysers, modern analyzers also use nondispersive infrared technology to detect a certain gas by detecting the absorption of infrared wavelengths that is characteristic of that gas. Infrared energy is emitted from a heated filament. By optically filtering the energy, the radiation spectrum is limited to the absorption band of the gas being measured. A detector measures the energy after the infrared energy has passed through the gas to be measured. This is compared to the energy at reference condition of no absorption.Many analysers are wall-mounted devices intended for long-term, unattended gas monitoring. There are now analysers that measure a range of gases and are highly portable to be suitable for a wider range of geoscience applications. Fast response high-precision analyzers are widely use to measure gas emissions and ecosystem fluxes using eddy covariance
Eddy covariance
The eddy covariance technique is a key atmospheric flux measurement technique to measure and calculate vertical turbulent fluxes within atmospheric boundary layers...
method when used together with fast-response sonic anemometer.
In some analysers, the reliability of measurements is enhanced by calibrating the analyser at the reference condition and a known span concentration. If the air would interfere with measurements, the chamber that houses the energy source is filled with a gas that has no detectable concentration of the gas being measured. Depending on the gas being measured, fresh air, chemically stripped air or nitrogen may be used.
See also
- Nondispersive infrared sensor
- ADC.
- LI-COR Biosciences - Portable research grade gas analyzers.
- Eddy covarianceEddy covarianceThe eddy covariance technique is a key atmospheric flux measurement technique to measure and calculate vertical turbulent fluxes within atmospheric boundary layers...

