
Hydrotrope
Encyclopedia
A hydrotrope is a compound that solubilises hydrophobic compounds in aqueous solutions. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (like surfactants) but the hydrophobic part is generally too small to cause spontaneous self-aggregation.
Hydrotropes do not have a critical concentration above which self-aggregation 'suddenly' starts to occur (as found for micelle
- and vesicle-forming surfactants, which have a critical micelle concentration
or cmc and a critical vesicle concentration or cvc, respectively). Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilisate has been added. Hydrotropes are in use industrially. Hydrotropes are used in detergent formulations to allow more concentrated formulations of surfactants. Examples of hydrotropes include sodium p-toluenesulfonate and sodium xylene sulfonate.
The term hydrotropy was originally put forward by Carl Neuberg
to describe the increase in the solubility of a solute by the addition of fairly high concentrations of alkali metal salts of various organic acids. However, the term has been used in the literature to designate non-micelle-forming substances, either liquids or solids, organic or inorganic, capable of solubilizing insoluble compounds.
The chemical structure of the conventional Neuberg’s hydrotropic salts (proto-type, sodium benzoate) consists generally of two essential parts, an anionic group and a hydrophobic aromatic ring or ring system. The anionic group is obviously involved in bringing about high aqueous solubility, which is a prerequisite for a hydrotropic substance. The type of anion or metal ion appeared to have a minor effect on the phenomenon. On the other hand, planarity of the hydrophobic part has been emphasized as an important factor in the mechanism of hydrotropic solubilization
Additives may either increase or decrease the solubility of a solute in a given solvent. These salts that increase solubility are said to ‘salt in’ the solute and those salts that decrease the solubility ‘salt out’ the solute. The effect of an additive depends very much on the influence, it has on the structure of water or its ability to compete with the solvent water molecules.
A convenient quantitation of the effect of a solute additive on the solubility of another solute may be obtained by the
Setschetow equation:
Hydrotropes do not have a critical concentration above which self-aggregation 'suddenly' starts to occur (as found for micelle
Micelle
A micelle is an aggregate of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single tail regions in the micelle centre. This phase is...
- and vesicle-forming surfactants, which have a critical micelle concentration
Critical micelle concentration
In colloidal and surface chemistry, the critical micelle concentration is defined as the concentration of surfactants above which micelles form and almost all additional surfactants added to the system go to micelles....
or cmc and a critical vesicle concentration or cvc, respectively). Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilisate has been added. Hydrotropes are in use industrially. Hydrotropes are used in detergent formulations to allow more concentrated formulations of surfactants. Examples of hydrotropes include sodium p-toluenesulfonate and sodium xylene sulfonate.
The term hydrotropy was originally put forward by Carl Neuberg
Carl Neuberg
Carl Alexander Neuberg was an early pioneer in biochemistry, and often referred to as the "Father of Biochemistry".He was the first editor of the journal Biochemische Zeitschrift. This journal was founded in 1906 and is now known as the FEBS Journal. Neuberg was born in Hanover, Germany and...
to describe the increase in the solubility of a solute by the addition of fairly high concentrations of alkali metal salts of various organic acids. However, the term has been used in the literature to designate non-micelle-forming substances, either liquids or solids, organic or inorganic, capable of solubilizing insoluble compounds.
The chemical structure of the conventional Neuberg’s hydrotropic salts (proto-type, sodium benzoate) consists generally of two essential parts, an anionic group and a hydrophobic aromatic ring or ring system. The anionic group is obviously involved in bringing about high aqueous solubility, which is a prerequisite for a hydrotropic substance. The type of anion or metal ion appeared to have a minor effect on the phenomenon. On the other hand, planarity of the hydrophobic part has been emphasized as an important factor in the mechanism of hydrotropic solubilization
Additives may either increase or decrease the solubility of a solute in a given solvent. These salts that increase solubility are said to ‘salt in’ the solute and those salts that decrease the solubility ‘salt out’ the solute. The effect of an additive depends very much on the influence, it has on the structure of water or its ability to compete with the solvent water molecules.
A convenient quantitation of the effect of a solute additive on the solubility of another solute may be obtained by the
Setschetow equation:
-
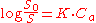 ; where
; where
- S0 = solubility in the absence of the additive
- S = solubility in the presence of the additive
- Ca = concentration of the additive
- K = salting coefficient, which is a measure of the sensitivity of the activity coefficient of the solute towards the salt.

