
Glucose 6-phosphatase
Encyclopedia
Glucose 6-phosphatase (Glc-6-Pase) is an enzyme
that hydrolyzes glucose-6-phosphate
resulting in the creation of a phosphate group and free glucose. Glucose is then exported from the cell via glucose transporter
membrane protein
s. This catalysis completes the final step in gluconeogenesis
and glycogenolysis
and therefore plays a key role in the homeostatic regulation of blood glucose levels. In humans, there are three isozymes, G6PC
, G6PC2
, and G6PC3
.
The Glc-6-Pase family includes two functional phosphohydrolases; Glc-6-Pase-α and Glc-6-Pase-β, the former of which is the prototype. Glc-6-Pase-α and Glc-6-Pase-β share similar active site structure, topology, mechanism of action, and kinetic properties with respect to Glc-6-P hydrolysis.
.png)
.png)
Glucose-6-Pase consists of 357 amino acids, and is anchored to the endoplasmic reticulum (ER) by nine transmembrane helicies. Its N-terminal and active site are found on the lumen side of the ER and its C-terminus projects into the cytoplasm. Due to its tight association to the ER, the exact structure of glucose-6-Pase remains unknown. However, sequence alignment has shown that glucose-6-Pase is structurally similar to the active site of the vanadium-containing chloroperoxidase found in Curvularia inaequalis.
Based on pH kinetic studies of Glc-6-Pase-α catalysis, it was proposed that the hydrolysis of Glucose-6-Phosphate was completed via a covalent phosphohistidine glucose-6-Phosphate intermediate. The active site of Glc-6-Pase-α was initially identified by the presence of a conserved phosphate signature motif usually found in lipid phosphatases, acid phosphatases, and vanadium haloperoxidases.
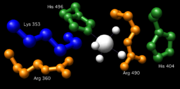
Essential residues in the active site of vanadium haloperoxidases include: Lys353, Arg360, Arg490, His404, and His496. Corresponding residues in the active site of Glc-6-Pase-α include Arg170 and Arg83, which donate hydrogen ions to the phosphate, stabilizing the transition state, His119, which provides a proton to the dephosphorylated oxygen attached to glucose, and His176, which completes a nucleophilic attack on the phosphate to form a covalently bound phosphoryl enzyme intermediate. Within the Vanadium-containing chloroperoxidase, Lys353 was found to stabilize the phosphate in the transition state. However, the corresponding residue in Glc-6-Pase-α (Lys76) resides within the ER membrane and its function, if any, is currently undetermined. With the exception of Lys76, these residues are all located on the luminal side of the ER membrane.
Glc-6-Pase-β is a ubiquitously expressed, 346-amino acid membrane protein that shares 36% sequence identity with Glc-6-Pase-α. Within the Glc-6-Pase-β enzyme, sequence alignments predict that its active site contains His167, His114, and Arg79. Similar to that of the Glc-6-Pase-α active site, His167 is the residue that provides the nucleophilic attack, and His114, and Arg79 are the hydrogen donors. Glc-6-Pase-β is also localized in the ER membrane, although its orientation is unknown.
unless it is dephosphorylated. The enzyme plays an important role during periods of fasting and when glucose levels are low. It has been shown that starvation and diabetes induces a 2-3-fold increase in Glc-6-Pase activity in the liver. Glc 6-Pase activity also increases dramatically at birth when an organism becomes independent of the mothers source of glucose. The human Glc 6-Pase gene contains five exons spanning approximately 125.5 kb DNA located on chromosome 17q21.
, which is characterized by loss of blood glucose homeostasis and disorders of glycogen and lipid metabolism.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that hydrolyzes glucose-6-phosphate
Glucose-6-phosphate
Glucose 6-phosphate is glucose sugar phosphorylated on carbon 6. This compound is very common in cells as the vast majority of glucose entering a cell will become phosphorylated in this way....
resulting in the creation of a phosphate group and free glucose. Glucose is then exported from the cell via glucose transporter
Glucose transporter
Glucose transporters are a wide group of membrane proteins that facilitate the transport of glucose over a plasma membrane. Because glucose is a vital source of energy for all life these transporters are present in all phyla...
membrane protein
Membrane protein
A membrane protein is a protein molecule that is attached to, or associated with the membrane of a cell or an organelle. More than half of all proteins interact with membranes.-Function:...
s. This catalysis completes the final step in gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
and glycogenolysis
Glycogenolysis
Glycogenolysis is the conversion of glycogen polymers to glucose monomers. Glycogen is catabolized by removal of a glucose monomer through cleavage with inorganic phosphate to produce glucose-1-phosphate...
and therefore plays a key role in the homeostatic regulation of blood glucose levels. In humans, there are three isozymes, G6PC
G6PC
Glucose-6-phosphatase is an enzyme that in humans is encoded by the G6PC gene.-Further reading:...
, G6PC2
G6PC2
Glucose-6-phosphatase 2 is an enzyme that in humans is encoded by the G6PC2 gene.-Further reading:...
, and G6PC3
G6PC3
Glucose-6-phosphatase 3, also known as glucose-6-phosphatase beta, is an enzyme that in humans is encoded by the G6PC3 gene.- Function :This gene encodes the catalytic subunit of glucose 6-phosphatase...
.
The Glc-6-Pase family includes two functional phosphohydrolases; Glc-6-Pase-α and Glc-6-Pase-β, the former of which is the prototype. Glc-6-Pase-α and Glc-6-Pase-β share similar active site structure, topology, mechanism of action, and kinetic properties with respect to Glc-6-P hydrolysis.
.png)
.png)

Structure and function
Although a clear consensus has not been reached, a large number of scientists adhere to a substrate-transport model to account for the catalytic properties of glucose-6-Pase. In this model, glucose 6-Pase has a low degree of selectivity. The transfer of the glucose 6-phosphate is carried out by a transporter protein (T1) and the endoplasmic reticulum (ER) contains structures allowing the exit of the phosphate group (T2) and glucose (T3).Glucose-6-Pase consists of 357 amino acids, and is anchored to the endoplasmic reticulum (ER) by nine transmembrane helicies. Its N-terminal and active site are found on the lumen side of the ER and its C-terminus projects into the cytoplasm. Due to its tight association to the ER, the exact structure of glucose-6-Pase remains unknown. However, sequence alignment has shown that glucose-6-Pase is structurally similar to the active site of the vanadium-containing chloroperoxidase found in Curvularia inaequalis.
Based on pH kinetic studies of Glc-6-Pase-α catalysis, it was proposed that the hydrolysis of Glucose-6-Phosphate was completed via a covalent phosphohistidine glucose-6-Phosphate intermediate. The active site of Glc-6-Pase-α was initially identified by the presence of a conserved phosphate signature motif usually found in lipid phosphatases, acid phosphatases, and vanadium haloperoxidases.
Essential residues in the active site of vanadium haloperoxidases include: Lys353, Arg360, Arg490, His404, and His496. Corresponding residues in the active site of Glc-6-Pase-α include Arg170 and Arg83, which donate hydrogen ions to the phosphate, stabilizing the transition state, His119, which provides a proton to the dephosphorylated oxygen attached to glucose, and His176, which completes a nucleophilic attack on the phosphate to form a covalently bound phosphoryl enzyme intermediate. Within the Vanadium-containing chloroperoxidase, Lys353 was found to stabilize the phosphate in the transition state. However, the corresponding residue in Glc-6-Pase-α (Lys76) resides within the ER membrane and its function, if any, is currently undetermined. With the exception of Lys76, these residues are all located on the luminal side of the ER membrane.
Glc-6-Pase-β is a ubiquitously expressed, 346-amino acid membrane protein that shares 36% sequence identity with Glc-6-Pase-α. Within the Glc-6-Pase-β enzyme, sequence alignments predict that its active site contains His167, His114, and Arg79. Similar to that of the Glc-6-Pase-α active site, His167 is the residue that provides the nucleophilic attack, and His114, and Arg79 are the hydrogen donors. Glc-6-Pase-β is also localized in the ER membrane, although its orientation is unknown.
Mechanism
The hydrolysis of Glc-6-P begins with a nucleophilic attack on the sugar-bound phosphate by His176 resulting in the formation of a phosphohistidine bond and the degradation of a carbonyl. A Negatively charged oxygen then transfers its electrons reforming a carbonyl and breaking its bond with glucose. The negatively charged glucose-bound oxygen is then protonated by His119 forming a free glucose. The phospho-intermediate produced by the reaction between His176 and the phosphate group is then broken by a hydrophilic attack; after the addition of another hydroxide and the decomposition of a carbonyl, the carbonyl is reformed kicking off the electrons originally donated by the His176 residue thereby creating a free phosphate group and completing the hydrolysis.Expression
Genes coding for the enzyme are primarily expressed in the liver, in the kidney cortex and (to a lesser extent) in the β-cells of the pancreatic islets and intestinal mucosa (especially during times of starvation). According to Surholt and Newsholme, Glc 6-Pase is present in a wide variety of muscles across the animal kingdom, albeit at very low concentrations. [1]. Thus, the glycogen that muscles store is not usually available for the rest of the body's cells because glucose-6-phosphate cannot cross the sarcolemmaSarcolemma
The sarcolemma is the cell membrane of a muscle cell . It consists of a true cell membrane, called the plasma membrane, and an outer coat made up of a thin layer of polysaccharide material that contains numerous thin collagen fibrils...
unless it is dephosphorylated. The enzyme plays an important role during periods of fasting and when glucose levels are low. It has been shown that starvation and diabetes induces a 2-3-fold increase in Glc-6-Pase activity in the liver. Glc 6-Pase activity also increases dramatically at birth when an organism becomes independent of the mothers source of glucose. The human Glc 6-Pase gene contains five exons spanning approximately 125.5 kb DNA located on chromosome 17q21.
Clinical significance
Mutations in Glc-6-Pase-α can lead to the glycogen storage disease type 1a called von Gierke's diseaseGlycogen storage disease type I
Glycogen storage disease type I or von Gierke's disease, is the most common of the glycogen storage diseases. This genetic disease results from deficiency of the enzyme glucose-6-phosphatase. This deficiency impairs the ability of the liver to produce free glucose from glycogen and from...
, which is characterized by loss of blood glucose homeostasis and disorders of glycogen and lipid metabolism.

