
Gamma-butyrobetaine dioxygenase
Encyclopedia
Gamma-butyrobetaine dioxygenase is an enzyme
that in humans is encoded by the BBOX1 gene
. Gamma-butyrobetaine dioxygenase catalyzes the formation of L-carnitine from gamma-butyrobetaine, the last step in the L-carnitine biosynthetic pathway. Carnitine is essential for the transport of activated fatty acids across the mitochondrial membrane during mitochondrial beta-oxidation.
The 3 substrates
of this enzyme are 4-trimethylammoniobutanoate, 2-oxoglutarate, and O2
, whereas its 3 products
are 3-hydroxy-4-trimethylammoniobutanoate, succinate, and CO2
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor. This enzyme participates in lysine degradation. It has 2 cofactors
: iron
, and Ascorbate.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that in humans is encoded by the BBOX1 gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
. Gamma-butyrobetaine dioxygenase catalyzes the formation of L-carnitine from gamma-butyrobetaine, the last step in the L-carnitine biosynthetic pathway. Carnitine is essential for the transport of activated fatty acids across the mitochondrial membrane during mitochondrial beta-oxidation.
Reaction
The following reaction is catalyzed by gamma-butyrobetaine dioxygenase:- 4-trimethylammoniobutanoate + 2-oxoglutarate + O2
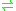 3-hydroxy-4-trimethylammoniobutanoate + succinate + CO2
3-hydroxy-4-trimethylammoniobutanoate + succinate + CO2
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are 4-trimethylammoniobutanoate, 2-oxoglutarate, and O2
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are 3-hydroxy-4-trimethylammoniobutanoate, succinate, and CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor. This enzyme participates in lysine degradation. It has 2 cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
: iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, and Ascorbate.

