
Foiled carbene
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a special type of stabilized carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
due to the proximity of a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
. This type of reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
is implicated in certain organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s. The positive interaction between carbene and double bond is only present in the singlet type and based on through-space electron transfer between the empty carbene p-orbital (LUMO
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
) and filled alkene double bond p-orbitals (HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
). The result is a three-center two-electron bond
Three-center two-electron bond
A three-center two-electron bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting in a...
akin to certain non-classical ion
Non-classical ion
Non-classical ions in organic chemistry are a special type of carbonium ions displaying delocalization of sigma bonds in 3-center-2-electron bonds of bridged systems. The term non-classical ion was first used by John D...
s.
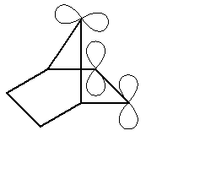
The increased stabilization blocks certain otherwise ordinary reaction modes for the carbene, hence the term foiled. An example is norbornen-7-ylidene, which is norbornene
Norbornene
Norbornene or norbornylene or norcamphene is a bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring bridged with a methylene group in the para position...
with a carbenic carbon atom at the bridge position. In silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
experiments reveal that the bridge in this molecule is actually bending towards the double bond with an optimum angle of around 90° vs 130° for norbornene.

