Excess molar quantity
Encyclopedia
Excess molar quantities are properties of mixtures which characterize the nonideal behaviour of real mixtures. They are the difference between the partial molar property of a component in a mixture and that of the pure component. The most frequently used excess molar quantities are the excess molar volume, excess molar enthalpies and heat capacities, excess chemical potential
.
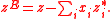
Here denotes the pure substance,
denotes the pure substance,  the excess molar property, and
the excess molar property, and  corresponds to the specific property under consideration. From the definition of partial molar properties,
corresponds to the specific property under consideration. From the definition of partial molar properties,

substitution yields:
Excess chemical potential
The excess chemical potential is defined as the difference between the chemical potential of a given species and that of an ideal gas under the same conditions ....
.
Definition
By definition, excess properties of a mixture are related to those of the pure substance by: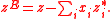
Here
 denotes the pure substance,
denotes the pure substance,  the excess molar property, and
the excess molar property, and  corresponds to the specific property under consideration. From the definition of partial molar properties,
corresponds to the specific property under consideration. From the definition of partial molar properties,
substitution yields: