
Erlenmeyer-Plöchl azlactone and amino acid synthesis
Encyclopedia
The Erlenmeyer-Plochl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer
who partly discovered the reaction, is a series of chemical reaction
s which transform glycine
to various other amino acid
s via an oxazolone
and an azlactone.
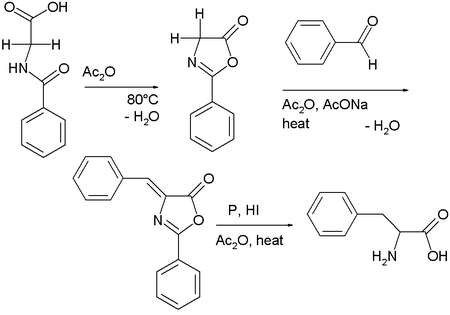 Hippuric acid
Hippuric acid
self-condenses in the presence of acetic anhydride
to 2-phenyl-oxazolone
. This intermediate also has two acidic protons and reacts with benzaldehyde
, acetic anhydride and sodium acetate to a so-called azlactone. This compound on reduction gives access to phenylalanine
.
Friedrich Gustav Carl Emil Erlenmeyer
Friedrich Gustav Carl Emil Erlenmeyer was a German chemist and the discoverer of the Erlenmeyer-Plöchl azlactone and amino acid synthesis. He was the son of Richard August Carl Emil Erlenmeyer....
who partly discovered the reaction, is a series of chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s which transform glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
to various other amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s via an oxazolone
Oxazolone
Oxazolone is a chemical allergen used for immunological experiments, particularly for experiments on delayed type hypersensitivity. Its long chemical name is 4-ethoxymethylene-2-phenyloxazol-5-one and it is commercially available.-References:...
and an azlactone.

Hippuric acid
Hippuric acid is a carboxylic acid found in the urine of horses and other herbivores. Hippuric acid crystallizes in rhombic prisms which are readily soluble in hot water, melt at 187 °C and decompose at about 240 °C...
self-condenses in the presence of acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
to 2-phenyl-oxazolone
Oxazolone
Oxazolone is a chemical allergen used for immunological experiments, particularly for experiments on delayed type hypersensitivity. Its long chemical name is 4-ethoxymethylene-2-phenyloxazol-5-one and it is commercially available.-References:...
. This intermediate also has two acidic protons and reacts with benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
, acetic anhydride and sodium acetate to a so-called azlactone. This compound on reduction gives access to phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
.
Scope
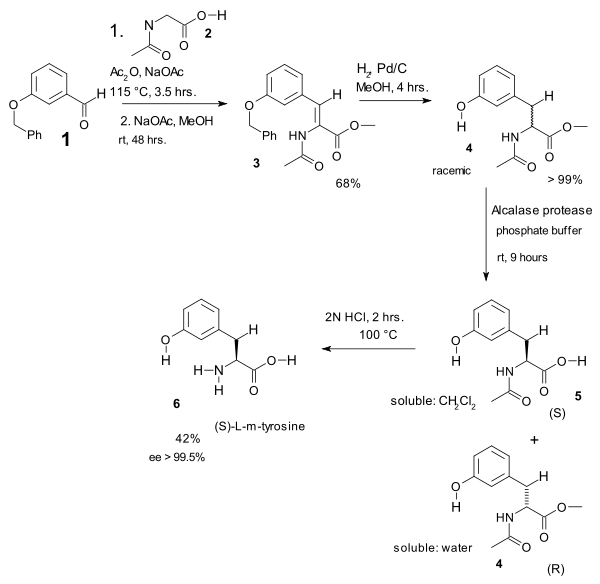
In one study the Erlenmeyer amino acid synthesis was used in the heart of an L-m-tyrosine synthesis
See also
- Dakin-West reactionDakin-West reactionThe Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin and Randolph West . Of special note, the keto-amide product is always racemic.With pyridine as a base and...
- Perkin reactionPerkin reactionThe Perkin reaction is an organic reaction developed by William Henry Perkin that can be used to make cinnamic acids i.e. α-β-unsaturated aromatic acid by the aldol condensation of aromatic aldehydes and acid anhydrides in the presence of an alkali salt of the acid.Several reviews have been written....

