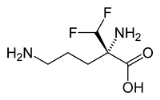
Eflornithine
Encyclopedia
Eflornithine is a drug
found to be effective in the treatment of facial hirsutism
(excessive hair growth) as well as in African trypanosomiasis (sleeping sickness). Eflornithine hydrochloride cream, which is for topical administration in women suffering from facial hirsutism, is marketed under the brand name Vaniqa by Almirall in Europe, CSL in Australia, Triton in Canada, Medison in Israel and SkinMedica in the USA. Eflornithine for injection against sleeping sickness is manufactured by Sanofi Aventis and sold under the brand name Ornidyl in the USA. Both are prescription drugs.
(FDA) granted a New Drug Application
for Vaniqa. The following year, the European Commission issued its Marketing Authorisation. Today, Vaniqa is marketed by Almirall in Europe, CSL in Australia, Triton in Canada, Medison in Israel and SkinMedica in the USA.
In 2001, Aventis (now Sanofi-Aventis) and the WHO formed a five-year partnership, during which more than 320,000 vials of pentamidine, over 420,000 vials of melarsoprol, and over 200,000 bottles of eflornithine were produced by Sanofi-Aventis, to be given to the WHO and distributed by the association Médecins sans Frontières
(also known as Doctors Without Borders) in countries where the sleeping sickness is endemic.
According to Médecins sans Frontières
, this only happened after "years of international pressure", and coinciding with the period when media attention was generated because of the launch of another eflornithine-based product (Vaniqa, geared to prevention of facial-hair in women), while its life-saving formulation (the one against sleeping sickness) was not being produced.
From 2001 (when production was restarted) through 2006, 14 million diagnoses were made. This greatly contributed to stemming the spread of sleeping sickness, and to saving nearly 110,000 lives. This changed the epidemiological profile of the disease, meaning that eliminating it altogether can now be envisaged.
(EC 4.1.1.17). This enzyme regulates cell division
by catalysing the first step in polyamine biosynthesis
. As the inhibitor has a low half-life in humans, it is broken down rapidly while the parasite cannot metabolise it quickly enough. This means that it preferentially harms the parasite.
affects between 5-15% of all women across all ethnic backgrounds. Depending on the definition and the underlying data, estimates indicate that approximately 40% of women have some degree of unwanted facial hair.
Hirsutism is usually the result of an underlying adrenal, ovarian, or central endocrine imbalance. Hirsutism is a commonly presenting symptom in dermatology, endocrinology and gynaecology clinics, and one that is considered to be the cause of much psychological distress and social difficulty. Facial hirsutism often leads to the avoidance of social situations and to symptoms of anxiety and depression.
Vaniqa is indicated for treatment of facial hirsutism in women. It is the only topical prescription treatment that slows the growth of facial hair. It is applied in a thin layer twice daily, a minimum of eight hours between applications. In clinical studies with Vaniqa, 81% percent of women showed clinical improvement after twelve months of treatment. Positive results were seen after eight weeks.
Vaniqa and laser treatment have complementary mechanisms of action. As Vaniqa does not affect hair diameter, it does not decrease the efficacy of simultaneous or subsequent laser therapy. Synergies between the two treatment methods lead to faster and better end results.
Vaniqa treatment significantly reduces the psychological burden of facial hirsutism.
Sleeping sickness is treated with pentamidine
in intramuscular injection in the first phase of the disease, and with melarsoprol
and eflornithine in intravenous injection (50 mg/kg every six hours for 14 days [7]) in the second phase of the disease.
Throughout clinical trials, data from a limited number of exposed pregnancies indicate that there is no clinical evidence that treatment with Vaniqa adversely affects pregnant women or foetuses.
Adequate and well-controlled studies with Ornidyl have not been performed regarding pregnancy in humans. Eflornithine should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus. However, since African trypanosomiasis has such a high mortality rate if left untreated, treatment with eflornithine may justify any potential risk to the fetus.
is thought to be due to a production defect rather than to peripheral destruction. Seizures were seen in approximately 8% of patients, but may be related to the disease state rather than the drug. Reversible hearing loss has occurred in 30–70% of patients receiving long-term therapy (more than 4–8 weeks of therapy or a total dose of >300 grams); high-frequency hearing is lost first, followed by middle- and low-frequency hearing. Because treatment for African trypanosomiasis is short-term, patients are unlikely to experience hearing loss.
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
found to be effective in the treatment of facial hirsutism
Hirsutism
Hirsutism or frazonism is the excessive hairiness on women in those parts of the body where terminal hair does not normally occur or is minimal - for example, a beard or chest hair. It refers to a male pattern of body hair and it is therefore primarily of cosmetic and psychological concern...
(excessive hair growth) as well as in African trypanosomiasis (sleeping sickness). Eflornithine hydrochloride cream, which is for topical administration in women suffering from facial hirsutism, is marketed under the brand name Vaniqa by Almirall in Europe, CSL in Australia, Triton in Canada, Medison in Israel and SkinMedica in the USA. Eflornithine for injection against sleeping sickness is manufactured by Sanofi Aventis and sold under the brand name Ornidyl in the USA. Both are prescription drugs.
History
Eflornithine was initially developed for cancer treatment at Merrell Dow Research Institute in the late 1970s, but, while having little use in treating malignancies, it was found to be highly effective in reducing hair growth, as well as in treatment of African trypanosomiasis (sleeping sickness), especially the West African form (Trypanosoma brucei gambiense).Hirsutism
In the 1980s, Gillette was awarded a patent for the discovery that topical application of eflornithine HCl cream inhibits hair growth. In the 1990s, Gillette conducted dose-ranging studies with eflornithine in hirsute women that demonstrated that the drug slows the rate of facial hair growth. Gillette then filed a patent for the formulation of eflornithine cream. In July 2000, the U.S. Food and Drug AdministrationFood and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
(FDA) granted a New Drug Application
New drug application
The New Drug Application is the vehicle in the United States through which drug sponsors formally propose that the Food and Drug Administration approve a new pharmaceutical for sale and marketing...
for Vaniqa. The following year, the European Commission issued its Marketing Authorisation. Today, Vaniqa is marketed by Almirall in Europe, CSL in Australia, Triton in Canada, Medison in Israel and SkinMedica in the USA.
Sleeping sickness treatment
The drug was registered for the treatment of gambiense sleeping sickness on November 28, 1990. However, in 1995 Aventis (now Sanofi-Aventis) stopped producing the drug, whose main market was African countries, because it didn't make a profit.In 2001, Aventis (now Sanofi-Aventis) and the WHO formed a five-year partnership, during which more than 320,000 vials of pentamidine, over 420,000 vials of melarsoprol, and over 200,000 bottles of eflornithine were produced by Sanofi-Aventis, to be given to the WHO and distributed by the association Médecins sans Frontières
Médecins Sans Frontières
' , or Doctors Without Borders, is a secular humanitarian-aid non-governmental organization best known for its projects in war-torn regions and developing countries facing endemic diseases. Its headquarters are in Geneva, Switzerland...
(also known as Doctors Without Borders) in countries where the sleeping sickness is endemic.
According to Médecins sans Frontières
Médecins Sans Frontières
' , or Doctors Without Borders, is a secular humanitarian-aid non-governmental organization best known for its projects in war-torn regions and developing countries facing endemic diseases. Its headquarters are in Geneva, Switzerland...
, this only happened after "years of international pressure", and coinciding with the period when media attention was generated because of the launch of another eflornithine-based product (Vaniqa, geared to prevention of facial-hair in women), while its life-saving formulation (the one against sleeping sickness) was not being produced.
From 2001 (when production was restarted) through 2006, 14 million diagnoses were made. This greatly contributed to stemming the spread of sleeping sickness, and to saving nearly 110,000 lives. This changed the epidemiological profile of the disease, meaning that eliminating it altogether can now be envisaged.
Hirsutism
Eflornithine topically applied is an irreversible inhibitor of ornithine decarboxylase (ODC), an enzyme that catalyses the conversion of ornithine to putrescine, which plays an important role in cell division and proliferation in the hair follicle.Sleeping sickness treatment
Eflornithine appears to kill trypanosomes by acting as a suicide inhibitor of the enzyme ornithine decarboxylaseOrnithine decarboxylase
The enzyme ornithine decarboxylase catalyzes the decarboxylation of ornithine to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer....
(EC 4.1.1.17). This enzyme regulates cell division
Cell division
Cell division is the process by which a parent cell divides into two or more daughter cells . Cell division is usually a small segment of a larger cell cycle. This type of cell division in eukaryotes is known as mitosis, and leaves the daughter cell capable of dividing again. The corresponding sort...
by catalysing the first step in polyamine biosynthesis
Polyamine
A polyamine is an organic compound having two or more primary amino groups .This class of compounds includes several synthetic substances that are important feedstocks for the chemical industry, such as ethylene diamine , 1,3-diaminopropane , and hexamethylenediamine...
. As the inhibitor has a low half-life in humans, it is broken down rapidly while the parasite cannot metabolise it quickly enough. This means that it preferentially harms the parasite.
Facial hirsutism in women
HirsutismHirsutism
Hirsutism or frazonism is the excessive hairiness on women in those parts of the body where terminal hair does not normally occur or is minimal - for example, a beard or chest hair. It refers to a male pattern of body hair and it is therefore primarily of cosmetic and psychological concern...
affects between 5-15% of all women across all ethnic backgrounds. Depending on the definition and the underlying data, estimates indicate that approximately 40% of women have some degree of unwanted facial hair.
Hirsutism is usually the result of an underlying adrenal, ovarian, or central endocrine imbalance. Hirsutism is a commonly presenting symptom in dermatology, endocrinology and gynaecology clinics, and one that is considered to be the cause of much psychological distress and social difficulty. Facial hirsutism often leads to the avoidance of social situations and to symptoms of anxiety and depression.
Vaniqa is indicated for treatment of facial hirsutism in women. It is the only topical prescription treatment that slows the growth of facial hair. It is applied in a thin layer twice daily, a minimum of eight hours between applications. In clinical studies with Vaniqa, 81% percent of women showed clinical improvement after twelve months of treatment. Positive results were seen after eight weeks.
Vaniqa and laser treatment have complementary mechanisms of action. As Vaniqa does not affect hair diameter, it does not decrease the efficacy of simultaneous or subsequent laser therapy. Synergies between the two treatment methods lead to faster and better end results.
Vaniqa treatment significantly reduces the psychological burden of facial hirsutism.
Sleeping sickness
Human African trypanosomiasis (HAT) is a parasitic disease spread by the tsetse fly. It is found only in subtropical and equatorial Africa. In 1995, the WHO estimated that 300,000 people were afflicted with the disease. In its 2001 report, the WHO set the figure of people at risk of infection at 60 million, of which only 4 to 5 million had access to any kind of medical monitoring. In 2006, the WHO estimated that about 70,000 people could have the disease, which, if left untreated, is always fatal.Sleeping sickness is treated with pentamidine
Pentamidine
Pentamidine is an antimicrobial medication given for prevention and treatment of Pneumocystis pneumonia caused by Pneumocystis jirovecii , a severe interstitial type of pneumonia often seen in patients with HIV infection...
in intramuscular injection in the first phase of the disease, and with melarsoprol
Melarsoprol
Melarsoprol is a medicinal drug used in the treatment of human African trypanosomiasis. It is also sold under the trade names “Mel B” and “Melarsen Oxide-BAL.”...
and eflornithine in intravenous injection (50 mg/kg every six hours for 14 days [7]) in the second phase of the disease.
Vaniqa
Vaniqa is a cream, which is white to off-white in colour. It is supplied in tubes of 30 g and 60 g in Europe. Vaniqa contains 15% w/w eflornithine hydrochloride monohydrate, corresponding to 11.5% w/w anhydrous eflornithine (EU), respectively 13.9% w/w anhydrous eflornithine hydrochloride (U.S.), in a cream for topical administration.Ornidyl
Ornidyl, intended for injection, is supplied in the strength of 200 mg eflornithine hydrochloride per ml.Vaniqa
Vaniqa is contraindicated for patients hypersensitive to eflornithine or to any of the excipients.Throughout clinical trials, data from a limited number of exposed pregnancies indicate that there is no clinical evidence that treatment with Vaniqa adversely affects pregnant women or foetuses.
Ornidyl
Ornidyl’s risk-benefit should be assessed in patients with impaired renal function or pre-existing hematologic abnormalities, as well as those with eighth-cranial-nerve impairment.Adequate and well-controlled studies with Ornidyl have not been performed regarding pregnancy in humans. Eflornithine should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus. However, since African trypanosomiasis has such a high mortality rate if left untreated, treatment with eflornithine may justify any potential risk to the fetus.
Adverse effects/side effects
Eflornithine is not genotoxic; no tumour-inducing effects have been observed in carcinogenicity studies, including one photocarcinogenicity study. No teratogenic effects have been detected.Topical Use
The most frequently reported side effect is acne (7-14%). Other side effects commonly (> 1%) reported are skin problems, such as skin reactions from in-growing hair, hair loss, burning, stinging or tingling sensations, dry skin, itching, redness or rash.Ornidyl
Most side effects related to systemic use of Ornidyl through injection are transient and reversible by discontinuing the drug or decreasing the dose. Hematologic abnormalities occur frequently, ranging from 10–55%. These abnormalities are dose-related and are usually reversible. ThrombocytopeniaThrombocytopenia
Thrombocytopenia is a relative decrease of platelets in blood.A normal human platelet count ranges from 150,000 to 450,000 platelets per microliter of blood. These limits are determined by the 2.5th lower and upper percentile, so values outside this range do not necessarily indicate disease...
is thought to be due to a production defect rather than to peripheral destruction. Seizures were seen in approximately 8% of patients, but may be related to the disease state rather than the drug. Reversible hearing loss has occurred in 30–70% of patients receiving long-term therapy (more than 4–8 weeks of therapy or a total dose of >300 grams); high-frequency hearing is lost first, followed by middle- and low-frequency hearing. Because treatment for African trypanosomiasis is short-term, patients are unlikely to experience hearing loss.
Ornidyl
Ornidyl may interact with bone marrow depressants and ototoxic medications.Vaniqa
Vaniqa, granted marketing approval by the US FDA, as well as by the European Commission among others, is currently the only topical prescription treatment that slows the growth of facial hair. Besides being a non-mechanical and non-cosmetic treatment, it is the only non-hormonal and non-systemic prescription option available for women who suffer from facial hirsutism. Vaniqa is marketed by Almirall in Europe, SkinMedica in the USA, Triton in Canada, Medison in Israel, and CSL in Australia.Ornidyl
The positive results of the 2001-2006 partnership between Sanofi-Aventis and the WHO in the fight against sleeping sickness motivated and justified the decision taken by the Sanofi-Aventis Group's senior management to continue supporting the WHO at the same level for another five years, 2006-2011.External links
- HairFacts: Vaniqa overview and medical data
- WHO Eflornithine page
- Almirall
- Sanofi Aventis
- Vaniqa
- Vaniqa (this site is intended for UK medical professionals only)
See also
- HirsutismHirsutismHirsutism or frazonism is the excessive hairiness on women in those parts of the body where terminal hair does not normally occur or is minimal - for example, a beard or chest hair. It refers to a male pattern of body hair and it is therefore primarily of cosmetic and psychological concern...
- African trypanosomiasis

