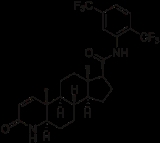
Dutasteride
Encyclopedia
Dutasteride is a dual 5-a reductase inhibitor
that inhibits conversion
of testosterone
to dihydrotestosterone
(DHT).
Dutasteride is FDA approved for treatment of benign prostatic hyperplasia
(BPH) and is also prescribed off-label for treatment of male pattern baldness (MPB).
(BPH) (also known as enlarged prostate).
The phase II results indicated that dutasteride at both 0.5 mg and 2.5 mg/day generated a superior hair count to finasteride 5 mg at 12 and 24 weeks.
Phase II study results at 24 weeks:
In December 2006, GlaxoSmithKline launched a new phase III, six month study in Korea
to test the safety, tolerability and effectiveness of a once-daily dose of dutasteride (0.5 mg) for the treatment of MPB in the vertex region of the scalp (types IIIV, IV and V on the Hamilton-Norwood scale
). The study was completed in January 2009. Future intentions by GlaxoSmithKline to continue or abandon plans for FDA approval of dutasteride in treatment of male pattern baldness (MPB) remain unknown.
, where a developing male child is naturally deficient in 5-alpha reductase type II, and thus unable to synthesize it. As dutasteride blocks the same process, developing males would have a DHT deficiency with its adverse effects as a result of the drug. Men who are taking dutasteride should not donate blood, and due to its long half-life, should also not donate blood for at least 6 months after the cessation of treatment. These precautions are to be taken in order to prevent the potential risk of causing birth defects in a pregnant woman who receives a transfusion with blood that contains dutasteride.
Month 7-12 ( n = 1,901 )
Month 13-18 ( n = 1,725 )
Month 19-24 ( n = 1,605 )
. Whilst the potential for positive, negative or neutral changes to the potential risk of developing prostate cancer with dutasteride has not been established, evidence has suggested it may temporarily reduce the growth and prevalence of benign prostate tumors, but could also mask the early detection of prostate cancer. The primary area for concern is for patients who may develop prostate cancer whilst taking dutasteride for benign prostatic hyperplasia, which in turn could delay diagnosis and early treatment of the prostate cancer, thereby potentially increasing the risk of these patients developing high-grade prostate cancer.
s, which block the action of the 5-alpha-reductase enzyme
s that convert testosterone into dihydrotestosterone (DHT). Finasteride
, which is also approved for the treatment of benign prostatic hyperplasia (BPH), in addition to the treatment of male pattern baldness (MPB), belongs to this class
of drugs
. Dutasteride inhibits both isoforms of 5-alpha reductase, type I and type II, whereas finasteride only inhibits type II. There are no long-term randomized trials comparing the effects of dutasteride and finasteride in patients with BPH. The EPICS trial, a 12-month clinical study done by GlaxoSmithKline, demonstrated treatment with dutasteride and finasteride resulted in similar decreases in prostate volume, with numerically but not statistically significantly greater improvements in symptom scores for the dutasteride group. Finasteride is marketed by Merck
under trademark names Proscar (5 mg/day finasteride) for BPH and Propecia (1 mg/day finasteride) for MPB. Published data from controlled clinical trials demonstrated the 5 mg dose of finasteride did not produce better results than the 1 mg dose.
5-alpha-reductase inhibitor
5α-Reductase inhibitors are a group of drugs with antiandrogenic activity, used in the treatment of benign prostatic hyperplasia and androgenic alopecia...
that inhibits conversion
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
of testosterone
Testosterone
Testosterone is a steroid hormone from the androgen group and is found in mammals, reptiles, birds, and other vertebrates. In mammals, testosterone is primarily secreted in the testes of males and the ovaries of females, although small amounts are also secreted by the adrenal glands...
to dihydrotestosterone
Dihydrotestosterone
Dihydrotestosterone is an androgen or male sex hormone. The enzyme 5α-reductase synthesises DHT in the prostate, testes, hair follicles, and adrenal glands...
(DHT).
Dutasteride is FDA approved for treatment of benign prostatic hyperplasia
Benign prostatic hyperplasia
Benign prostatic hyperplasia also known as benign prostatic hypertrophy , benign enlargement of the prostate , and adenofibromyomatous hyperplasia, refers to the increase in size of the prostate....
(BPH) and is also prescribed off-label for treatment of male pattern baldness (MPB).
Benign prostatic hyperplasia
Dutasteride is approved for the treatment of benign prostatic hyperplasiaBenign prostatic hyperplasia
Benign prostatic hyperplasia also known as benign prostatic hypertrophy , benign enlargement of the prostate , and adenofibromyomatous hyperplasia, refers to the increase in size of the prostate....
(BPH) (also known as enlarged prostate).
Male pattern baldness
Phase I and II clinical trials for dutasteride as a hair loss drug were undertaken, but called off in late 2002, with reasons for terminatation unknown.The phase II results indicated that dutasteride at both 0.5 mg and 2.5 mg/day generated a superior hair count to finasteride 5 mg at 12 and 24 weeks.
Phase II study results at 24 weeks:
- Placebo: - 32.3 hairs
- Finasteride 5 mg: + 75.6 hairs
- Dutasteride 0.1 mg: + 78.5 hairs
- Dutasteride 0.5 mg: + 94.6 hairs
- Dutasteride 2.5 mg: + 109.6 hairs
In December 2006, GlaxoSmithKline launched a new phase III, six month study in Korea
Korea
Korea ) is an East Asian geographic region that is currently divided into two separate sovereign states — North Korea and South Korea. Located on the Korean Peninsula, Korea is bordered by the People's Republic of China to the northwest, Russia to the northeast, and is separated from Japan to the...
to test the safety, tolerability and effectiveness of a once-daily dose of dutasteride (0.5 mg) for the treatment of MPB in the vertex region of the scalp (types IIIV, IV and V on the Hamilton-Norwood scale
Hamilton-Norwood scale
The progression of male pattern baldness is generally classified on the Hamilton-Norwood scale, which ranges from stages I to VII.This measurement scale was first introduced by Dr. James Hamilton in the 1950s and later revised and updated by Dr. O'Tar Norwood in the 1970s....
). The study was completed in January 2009. Future intentions by GlaxoSmithKline to continue or abandon plans for FDA approval of dutasteride in treatment of male pattern baldness (MPB) remain unknown.
Contraindications
The teratogenic effect (abnormalities of physiological development) from dutasteride is harmful to male children. Women who are pregnant should not handle the capsules, as inadvertent consumption, such as skin contact, could cause birth defects of the male fetus. The adverse effects would be similar to 5-alpha-reductase deficiency5-alpha-reductase deficiency
5-Alpha-reductase deficiency is an autosomal recessive intersex condition caused by a mutation of the 5-alpha reductase type 2 gene.-Normal function:...
, where a developing male child is naturally deficient in 5-alpha reductase type II, and thus unable to synthesize it. As dutasteride blocks the same process, developing males would have a DHT deficiency with its adverse effects as a result of the drug. Men who are taking dutasteride should not donate blood, and due to its long half-life, should also not donate blood for at least 6 months after the cessation of treatment. These precautions are to be taken in order to prevent the potential risk of causing birth defects in a pregnant woman who receives a transfusion with blood that contains dutasteride.
Clinical trial results
Month 0-6 ( n = 2,167 )- Impotence: 4.7%
- Decreased libido: 3%
- Ejaculation disorders: 1.4%
- Breast disorders: 0.5%
Month 7-12 ( n = 1,901 )
- Impotence: 1.4%
- Decreased libido: 0.3%
- Ejaculation disorders: 0.5%
- Breast disorders: 1.1%
Month 13-18 ( n = 1,725 )
- Impotence: 1%
- Decreased libido: 0.1%
- Ejaculation disorders: 0.4%
- Breast disorders: 0.8%
Month 19-24 ( n = 1,605 )
- Impotence: 0.8%
- Decreased libido: 0.3%
- Ejaculation disorders: 0.1%
- Breast disorders: 0.6%
Observed in practice
The FDA has added warning to dutasteride about an increased risk of high-grade prostate cancerProstate cancer
Prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Most prostate cancers are slow growing; however, there are cases of aggressive prostate cancers. The cancer cells may metastasize from the prostate to other parts of the body, particularly...
. Whilst the potential for positive, negative or neutral changes to the potential risk of developing prostate cancer with dutasteride has not been established, evidence has suggested it may temporarily reduce the growth and prevalence of benign prostate tumors, but could also mask the early detection of prostate cancer. The primary area for concern is for patients who may develop prostate cancer whilst taking dutasteride for benign prostatic hyperplasia, which in turn could delay diagnosis and early treatment of the prostate cancer, thereby potentially increasing the risk of these patients developing high-grade prostate cancer.
Mechanism of action
Dutasteride belongs to a class of drugs called 5-alpha-reductase inhibitor5-alpha-reductase inhibitor
5α-Reductase inhibitors are a group of drugs with antiandrogenic activity, used in the treatment of benign prostatic hyperplasia and androgenic alopecia...
s, which block the action of the 5-alpha-reductase enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s that convert testosterone into dihydrotestosterone (DHT). Finasteride
Finasteride
Finasteride is a synthetic antiandrogen that inhibits type II 5-alpha reductase, the enzyme that converts testosterone to dihydrotestosterone...
, which is also approved for the treatment of benign prostatic hyperplasia (BPH), in addition to the treatment of male pattern baldness (MPB), belongs to this class
Chemical classification
Chemical classification systems attempt to classify as elements or compounds according to certain chemical functional or structural properties. Whereas the structural properties are largely intrinsic, functional properties and the derived classifications depend to a certain degree on the type of...
of drugs
DRUGS
Destroy Rebuild Until God Shows are an American post-hardcore band formed in 2010. They released their debut self-titled album on February 22, 2011.- Formation :...
. Dutasteride inhibits both isoforms of 5-alpha reductase, type I and type II, whereas finasteride only inhibits type II. There are no long-term randomized trials comparing the effects of dutasteride and finasteride in patients with BPH. The EPICS trial, a 12-month clinical study done by GlaxoSmithKline, demonstrated treatment with dutasteride and finasteride resulted in similar decreases in prostate volume, with numerically but not statistically significantly greater improvements in symptom scores for the dutasteride group. Finasteride is marketed by Merck
Merck & Co.
Merck & Co., Inc. , also known as Merck Sharp & Dohme or MSD outside the United States and Canada, is one of the largest pharmaceutical companies in the world. The Merck headquarters is located in Whitehouse Station, New Jersey, an unincorporated area in Readington Township...
under trademark names Proscar (5 mg/day finasteride) for BPH and Propecia (1 mg/day finasteride) for MPB. Published data from controlled clinical trials demonstrated the 5 mg dose of finasteride did not produce better results than the 1 mg dose.

