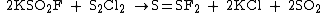Disulfur difluoride
Encyclopedia
Disulfur difluoride is a halide
of sulfur
, with the chemical formula S2F2. Silver(II) fluoride
can fluorinate sulfur in a strictly dry container, and the reaction produces FS-SF:
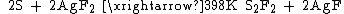
Disulfur difluoride will undergo intramolecular rearrangement with the existence of alkali elements' fluorides, obtaining the isomer S=SF2:
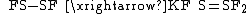
S=SF2 can be synthesized with the reaction of potassium fluorosulfinate and sulfur dichloride
:
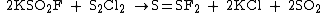
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, with the chemical formula S2F2. Silver(II) fluoride
Silver(II) fluoride
Silver fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver compound. Silver is usually present in its +1 oxidation state. It is used as a fluorinating agent.-Preparation:...
can fluorinate sulfur in a strictly dry container, and the reaction produces FS-SF:
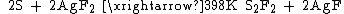
Disulfur difluoride will undergo intramolecular rearrangement with the existence of alkali elements' fluorides, obtaining the isomer S=SF2:
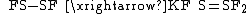
S=SF2 can be synthesized with the reaction of potassium fluorosulfinate and sulfur dichloride
Sulfur dichloride
Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common. It is used as a precursor to organosulfur compounds.-Chlorination of sulfur:...
: