Cytochrome b5
Encyclopedia
Cytochromes b5 are ubiquitous electron transport hemoprotein
s found in animal
s, plant
s, fungi and purple phototrophic bacteria
. The microsomal
and mitochondrial variants are membrane-bound, while bacterial and those from erythrocytes and other animal tissues are water-soluble. The family of cytochrome b5-like proteins includes (besides cytochrome b5 itself) hemoprotein domains covalently associated with other redox domains in flavocytochrome cytochrome b2 (L-lactate dehydrogenase; ), sulfite oxidase , plant and fungal nitrate reductases , and plant and fungal cytochrome b5/acyl lipid desaturase fusion proteins.
residues provide the fifth and sixth heme ligands, and the propionate edge of the heme group lies at the opening of the heme crevice. Two isomers of cytochrome b5, referred to as the A (major) and B (minor) forms, differ by a 180° rotation of the heme about an axis defined by the α- and γ-meso carbons.
L-ascorbate—cytochrome-b5 reductase
CMP-N-acetylneuraminate monooxygenase
stearoyl-CoA 9-desaturase
linoleoyl-CoA 9-desaturase
Hemoprotein
A hemeprotein , or heme protein, is a metalloprotein containing a heme prosthetic group- an organic compound that allows a protein to carry out a function that it cannot do alone....
s found in animal
Animal
Animals are a major group of multicellular, eukaryotic organisms of the kingdom Animalia or Metazoa. Their body plan eventually becomes fixed as they develop, although some undergo a process of metamorphosis later on in their life. Most animals are motile, meaning they can move spontaneously and...
s, plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s, fungi and purple phototrophic bacteria
Purple bacteria
Purple bacteria or purple photosynthetic bacteria are proteobacteria that are phototrophic, that is capable of producing energy through photosynthesis...
. The microsomal
Microsome
In cell biology, microsomes are vesicle-like artifacts re-formed from pieces of the endoplasmic reticulum when eukaryotic cells are broken-up in the laboratory; by definition, microsomes are not ordinarily present in living cells....
and mitochondrial variants are membrane-bound, while bacterial and those from erythrocytes and other animal tissues are water-soluble. The family of cytochrome b5-like proteins includes (besides cytochrome b5 itself) hemoprotein domains covalently associated with other redox domains in flavocytochrome cytochrome b2 (L-lactate dehydrogenase; ), sulfite oxidase , plant and fungal nitrate reductases , and plant and fungal cytochrome b5/acyl lipid desaturase fusion proteins.
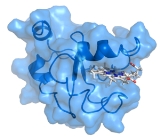
Structure
3-D structures of a number of cytochrome b5 and yeast flavocytochrome b2 are known. The fold belongs to the α+β class, with two hydrophobic cores on each side of a β-sheet. The larger hydrophobic core constitutes the heme-binding pocket, closed off on each side by a pair of helices connected by a turn. The smaller hydrophobic core may have only a structural role and is formed by spatially close N-terminal and C-terminal segments. The two histidineHistidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues provide the fifth and sixth heme ligands, and the propionate edge of the heme group lies at the opening of the heme crevice. Two isomers of cytochrome b5, referred to as the A (major) and B (minor) forms, differ by a 180° rotation of the heme about an axis defined by the α- and γ-meso carbons.
Cytochrome b5 in some biochemical reactions
cytochrome-b5 reductaseCytochrome b5 reductase
Cytochrome-b5 reductase is a NADH-dependent enzyme that converts methemoglobin to hemoglobin...
- NADH + H+ + 2 ferricytochrome b5 = NAD+ + 2 ferrocytochrome b5
L-ascorbate—cytochrome-b5 reductase
L-ascorbate-cytochrome-b5 reductase
In enzymology, a L-ascorbate—cytochrome-b5 reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are L-ascorbate and ferricytochrome b5, whereas its 3 products are monodehydroascorbate, ferrocytochrome b5, and H+....
- L-ascorbate + ferricytochrome b5 = monodehydroascorbate + ferrocytochrome b5
CMP-N-acetylneuraminate monooxygenase
CMP-N-acetylneuraminate monooxygenase
In enzymology, a CMP-N-acetylneuraminate monooxygenase is an enzyme that catalyzes the chemical reactionThe 4 substrates of this enzyme are CMP-N-acetylneuraminate, ferrocytochrome b5, O2, and H+, whereas its 3 products are CMP-N-glycoloylneuraminate, ferricytochrome b5, and H2O.This enzyme...
- CMP-N-acetylneuraminate + 2 ferrocytochrome b5 + O2 + 2 H+ = CMP-N-glycoloylneuraminate + 2 ferricytochrome b5 + H2O
stearoyl-CoA 9-desaturase
Stearoyl-CoA 9-desaturase
In enzymology, a stearoyl-CoA 9-desaturase is an enzyme that catalyzes the chemical reactionThe 4 substrates of this enzyme are stearoyl-CoA, ferrocytochrome b5, O2, and H+, whereas its 3 products are oleoyl-CoA, ferricytochrome b5, and H2O....
- stearoyl-CoA + 2 ferrocytochrome b5 + O2 + 2 H+ = oleoyl-CoA + 2 ferricytochrome b5 + H2O
linoleoyl-CoA 9-desaturase
- linoleoyl-CoA + 2 ferrocytochrome b5 + O2 + 2 H+ = γ-linolenoyl-CoA + 2 ferricytochrome b5 + H2O

