Chloramphenicol acetyltransferase
Encyclopedia
Chloramphenicol acetyltransferase (or CAT) is a bacteria
l enzyme
that detoxifies the antibiotic
chloramphenicol
and is responsible for chloramphenicol resistance in bacteria. This enzyme covalently attaches an acetyl group from acetyl-CoA
to chloramphenicol, which prevents chloramphenicol from binding to ribosome
s. A histidine residue, located in the C-terminal section of the enzyme, plays a central role in its catalytic mechanism.
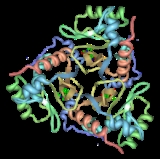
The crystal structure of the type III enzyme from Escherichia coli
with chloramphenicol bound has been determined. CAT is a trimer of identical subunits (monomer Mr 25,000) and the trimeric structure is stabilised by a number of hydrogen bonds, some of which result in the extension of a beta-sheet across the subunit interface. Chloramphenicol binds in a deep pocket located at the boundary between adjacent subunits of the trimer, such that the majority of residues forming the binding pocket belong to one subunit while the catalytically essential histidine belongs to the adjacent subunit. His195 is appropriately positioned to act as a general base catalyst in the reaction, and the required tautomeric stabilisation is provided by an unusual interaction with a main-chain carbonyl oxygen.
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
l enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that detoxifies the antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
chloramphenicol
Chloramphenicol
Chloramphenicol is a bacteriostatic antimicrobial that became available in 1949. It is considered a prototypical broad-spectrum antibiotic, alongside the tetracyclines, and as it is both cheap and easy to manufacture it is frequently found as a drug of choice in the third world.Chloramphenicol is...
and is responsible for chloramphenicol resistance in bacteria. This enzyme covalently attaches an acetyl group from acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
to chloramphenicol, which prevents chloramphenicol from binding to ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
s. A histidine residue, located in the C-terminal section of the enzyme, plays a central role in its catalytic mechanism.
The crystal structure of the type III enzyme from Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
with chloramphenicol bound has been determined. CAT is a trimer of identical subunits (monomer Mr 25,000) and the trimeric structure is stabilised by a number of hydrogen bonds, some of which result in the extension of a beta-sheet across the subunit interface. Chloramphenicol binds in a deep pocket located at the boundary between adjacent subunits of the trimer, such that the majority of residues forming the binding pocket belong to one subunit while the catalytically essential histidine belongs to the adjacent subunit. His195 is appropriately positioned to act as a general base catalyst in the reaction, and the required tautomeric stabilisation is provided by an unusual interaction with a main-chain carbonyl oxygen.

