CX717
Encyclopedia
CX717 is an ampakine
compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals
. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.
(IND) application to initiate pilot Phase II clinical trials in the United States
.
Also, in 2005, the United States Department of Defense
funded a study to look into CX717 and the physiological effects of sleepiness. The study found that rhesus monkeys performed faster and better after receiving the drug, and it counteracted the effects of sleep deprivation.
However, a 2006 study funded by DARPA found that CX717 did not improve cognitive performance in humans subjected to simulated night shift work.
In early March 2006 Cortex reported that, in a small pilot Phase II study, CX717 had demonstrated positive clinical and statistical results on the primary endpoint, the ADHD rating scale and the sub-scales related to attention and hyperactivity which are used for the approval of all currently available ADHD treatments. According to a Cortex Pharmaceuticals press release, "Consistent with all previous studies involving over 220 patients and healthy adults, this study demonstrated that CX717 was safe, well tolerated, and produced no increase in heart rate, blood pressure or other cardiovascular side effects".
In April 2007 Cortex Pharmaceuticals submitted two large data packages to the FDA regarding CX717. One data set went to the FDA's Division of Neurology Drug Products for the treatment of Alzheimer's disease
, while the other went to the Division of Psychiatry Products where the company filed a second CX717 IND for the treatment of ADHD. According to a Cortex Pharmaceuticals press release, the submitted data package "provides clear evidence that the specific histopathological changes seen in animal toxicology studies, which previously caused the FDA to put CX717 on clinical hold, is a postmortem fixation artifact and is not found in the tissue of the animal when it is still living".
Roger G Stoll PhD, Chief Executive Officer of Cortex, stated,
However, in October 2007 the FDA denied Cortex's IND application for a Phase IIb study of CX717 for treatment of ADHD, based on the same animal toxicology results. Cortex responded by inactivating the application, although it will "continue its plans to develop CX717 for the acute treatment of respiratory depression (RD) and continue its study of CX717 in its Alzheimer’s disease PET scan study. Cortex believes that the IND application previously filed with the Division of Neurology Products of the FDA for the treatment of Alzheimer’s disease will not be affected by the actions of the DPP." The company hopes that after the use of the compound in treating a high-risk acute condition is approved and well-established, the risks of longer-term use at higher doses, such as for treatment of ADHD, will be shown to be less than the FDA had concluded.
effects of the ampakine drugs on the pre-Botzinger complex
of the brain has led to continued development of an intravenous formulation of CX-717 for use alongside opioid
analgesics, along with an oral formulation of CX-1739, which is around 3-5x more potent than CX-717 and has better oral bioavailability, and is being trialled for treatment of sleep apnoea. Further research has investigated the neurological mechanisms behind the anti-respiratory depressant effects of CX-717, and demonstrated that it can be used in humans alongside opioid drugs to reduce this side effect without affecting analgesia.
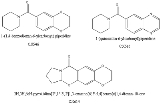
and ADHD. These drugs were reasonably effective at reducing the symptoms of Alzheimer's and it was hoped that they could also slow the progression of the disease, but both CX-546 and CX-614 have poor bioavailability, and are only active at very high doses of 1000 mg or more. CX-717 and CX-1739 are newer and more potent drugs in the same series. The chemical structures of CX-717 and CX-1739 have not yet been revealed by Cortex Pharmaceuticals, but are presumably similar to earlier compounds in the series as shown below. It is very unusual for research on a compound to be released in scientific journals without disclosing exactly what the compound consists of, but this information is likely to have been kept confidential for reasons of intellectual property, and also because the research on CX-717 was initially partially funded by DARPA, the United States Defense Advanced Research Projects Agency.
Ampakine
Ampakines are a class of compounds known to enhance attention span and alertness, and facilitate learning and memory. The ampakines take their name from the glutamatergic AMPA receptor with which they strongly interact...
compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals
Cortex Pharmaceuticals
Cortex Pharmaceuticals is a pharmaceutical company based in Irvine, California specializing in positive allosteric modulators of the AMPA receptor known as Ampakines.-History:February 2005 – Cortex received U.S...
. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.
Approval process
In 2005 the U.S. Food and Drug Administration (FDA) accepted Cortex Pharmaceuticals' Investigational New DrugInvestigational New Drug
The United States Food and Drug Administration's Investigational New Drug program is the means by which a pharmaceutical company obtains permission to ship an experimental drug across state lines before a marketing application for the drug has been approved...
(IND) application to initiate pilot Phase II clinical trials in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
.
Also, in 2005, the United States Department of Defense
United States Department of Defense
The United States Department of Defense is the U.S...
funded a study to look into CX717 and the physiological effects of sleepiness. The study found that rhesus monkeys performed faster and better after receiving the drug, and it counteracted the effects of sleep deprivation.
However, a 2006 study funded by DARPA found that CX717 did not improve cognitive performance in humans subjected to simulated night shift work.
In early March 2006 Cortex reported that, in a small pilot Phase II study, CX717 had demonstrated positive clinical and statistical results on the primary endpoint, the ADHD rating scale and the sub-scales related to attention and hyperactivity which are used for the approval of all currently available ADHD treatments. According to a Cortex Pharmaceuticals press release, "Consistent with all previous studies involving over 220 patients and healthy adults, this study demonstrated that CX717 was safe, well tolerated, and produced no increase in heart rate, blood pressure or other cardiovascular side effects".
In April 2007 Cortex Pharmaceuticals submitted two large data packages to the FDA regarding CX717. One data set went to the FDA's Division of Neurology Drug Products for the treatment of Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
, while the other went to the Division of Psychiatry Products where the company filed a second CX717 IND for the treatment of ADHD. According to a Cortex Pharmaceuticals press release, the submitted data package "provides clear evidence that the specific histopathological changes seen in animal toxicology studies, which previously caused the FDA to put CX717 on clinical hold, is a postmortem fixation artifact and is not found in the tissue of the animal when it is still living".
Roger G Stoll PhD, Chief Executive Officer of Cortex, stated,
“When CX717 was removed from clinical hold on October 6, 2006 by the Neurology Division a dose was permitted for continuing a study in patients with Alzheimer's diseaseAlzheimer's diseaseAlzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
, but that dose was too low to permit the assessment of the drug in patients with ADHD. Further information was needed to better understand the cause of the histopathological changes. We now have a substantial data base which clearly documents the fact that the histological changes of concern occur postmortem when the fixative solution is used to prepare the slides of the tissue specimens.”
However, in October 2007 the FDA denied Cortex's IND application for a Phase IIb study of CX717 for treatment of ADHD, based on the same animal toxicology results. Cortex responded by inactivating the application, although it will "continue its plans to develop CX717 for the acute treatment of respiratory depression (RD) and continue its study of CX717 in its Alzheimer’s disease PET scan study. Cortex believes that the IND application previously filed with the Division of Neurology Products of the FDA for the treatment of Alzheimer’s disease will not be affected by the actions of the DPP." The company hopes that after the use of the compound in treating a high-risk acute condition is approved and well-established, the risks of longer-term use at higher doses, such as for treatment of ADHD, will be shown to be less than the FDA had concluded.
Use for reversal of respiratory depression
The relatively poor oral bioavailability and blood-brain barrier penetration of CX-717 ultimately led to Cortex abandoning development of the 800 mg oral formulation of CX-717 for ADHD, although research into its action in the brain continues. However the unexpected discovery of the strong respiratory stimulantRespiratory stimulant
A respiratory stimulant is a drug which acts to increase the action of the respiratory system.An example is doxapram....
effects of the ampakine drugs on the pre-Botzinger complex
Pre-Botzinger complex
The Pre-Bötzinger Complex is a cluster of interneurons in the ventrolateral medulla of the brainstem, which is essential to the generation of respiratory rhythm in mammals...
of the brain has led to continued development of an intravenous formulation of CX-717 for use alongside opioid
Opioid
An opioid is a psychoactive chemical that works by binding to opioid receptors, which are found principally in the central and peripheral nervous system and the gastrointestinal tract...
analgesics, along with an oral formulation of CX-1739, which is around 3-5x more potent than CX-717 and has better oral bioavailability, and is being trialled for treatment of sleep apnoea. Further research has investigated the neurological mechanisms behind the anti-respiratory depressant effects of CX-717, and demonstrated that it can be used in humans alongside opioid drugs to reduce this side effect without affecting analgesia.
Related AMPAkines
Other AMPAkine drugs from Cortex Pharmaceuticals such as CX-546 and CX-614 have already been researched for use in treating Alzheimer's diseaseAlzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
and ADHD. These drugs were reasonably effective at reducing the symptoms of Alzheimer's and it was hoped that they could also slow the progression of the disease, but both CX-546 and CX-614 have poor bioavailability, and are only active at very high doses of 1000 mg or more. CX-717 and CX-1739 are newer and more potent drugs in the same series. The chemical structures of CX-717 and CX-1739 have not yet been revealed by Cortex Pharmaceuticals, but are presumably similar to earlier compounds in the series as shown below. It is very unusual for research on a compound to be released in scientific journals without disclosing exactly what the compound consists of, but this information is likely to have been kept confidential for reasons of intellectual property, and also because the research on CX-717 was initially partially funded by DARPA, the United States Defense Advanced Research Projects Agency.
See also
- AdrafinilAdrafinilAdrafinil is a mild central nervous system stimulant drug used to relieve excessive sleepiness and inattention in elderly patients...
- AMPAAMPAAMPA is a compound that is a specific agonist for the AMPA receptor, where it mimics the effects of the neurotransmitter glutamate....
- ArecolineArecolineArecoline is an alkaloid natural product found in the areca nut, the fruit of the areca palm . It is an odourless oily liquid volatile in steam, miscible with most organic solvents and water, but extractable from the latter by ether in presence of dissolved salts. The salts are crystalline, but...
- CarphedonCarphedonPhenotropil is a derivative of the nootropic drug -4-pheyl-2-pyrrolidon. It was developed in Russia, and a small number of low-scale clinical studies have shown possible links between prescription of carphedon and improvement in a number of encephalopathic conditions, including lesions of cerebral...
- CX-516CX-516CX-516 is an ampakine and nootropic that acts as an AMPA receptor positive allosteric modulator and is being developed by a collaboration of Cortex and Shire and Servier...
(Ampalex) - CX-546CX546CX-546 is an ampakine drug developed by Cortex Pharmaceuticals.It has been proposed as a treatment for schizophrenia. CX-546 was the second drug of note to come out of the Cortex research program, after CX-516, but while it was an improvement over its predecessor in some respects, it still has...
- CX-614CX614CX-614 is an ampakine drug developed by Cortex Pharmaceuticals. It has been investigated for its effect on AMPA receptors.Chronic CX-614 treatments produce rapid increases in the synthesis of the brain-derived neurotrophic factor BDNF which has very important effects on synaptic plasticity and may...
- DesmopressinDesmopressinDesmopressin is a synthetic replacement for vasopressin, the hormone that reduces urine production. It may be taken nasally, intravenously, or as a tablet...
- FarampatorFarampatorFarampator is an ampakine drug. It was developed by Cortex Pharmaceuticals, and licensed to Organon BioSciences for commercial development...
- IdebenoneIdebenoneIdebenone is an experimental drug, initially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer's disease and other cognitive defects. This has been met with limited success. The Swiss company Santhera Pharmaceuticals has started to investigate it for the treatment of...
- ModafinilModafinilModafinil is an analeptic drug manufactured by Cephalon, and is approved by the U.S. Food and Drug Administration for the treatment of narcolepsy, shift work sleep disorder, and excessive daytime sleepiness associated with obstructive sleep apnea...
- VasopressinVasopressinArginine vasopressin , also known as vasopressin, argipressin or antidiuretic hormone , is a neurohypophysial hormone found in most mammals, including humans. Vasopressin is a peptide hormone that controls the reabsorption of molecules in the tubules of the kidneys by affecting the tissue's...
- VinpocetineVinpocetineVinpocetine is a semisynthetic derivative alkaloid of vincamine , an extract from the periwinkle plant....

