
Buckingham potential
Encyclopedia
The Buckingham potential is a formula that describes the Pauli repulsion energy and van der Waals energy 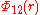 for the interaction of two atoms that are not directly bonded as a function of the interatomic distance
for the interaction of two atoms that are not directly bonded as a function of the interatomic distance  .
.

Here, ,
,  and
and  are constants. The two terms on the right hand side constitute a repulsion and an attraction, because they are positive and negative, respectively.
are constants. The two terms on the right hand side constitute a repulsion and an attraction, because they are positive and negative, respectively.
Richard A. Buckingham proposed this, as a simplification of the Lennard-Jones potential
, in a theoretical study of the equation of state for gaseous helium, neon and argon
As explained in Buckingham's original paper and, e.g., in section 2.2.5 of Jensen's text the repulsion is due to the interpenetration of the closed electron shells. "There is therefore some justification for choosing the repulsive part (of the potential) as an exponential function." The Buckingham potential has been used extensively in simulations of molecular dynamics.
Because the exponential term converges to a constant as →
→ , while the
, while the 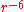 term diverges, the Buckingham potential "turns over" as
term diverges, the Buckingham potential "turns over" as  becomes small. This may be problematic when dealing with a structure with very short interatomic distances, as "nuclear fusion" can occur.
becomes small. This may be problematic when dealing with a structure with very short interatomic distances, as "nuclear fusion" can occur.
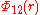 for the interaction of two atoms that are not directly bonded as a function of the interatomic distance
for the interaction of two atoms that are not directly bonded as a function of the interatomic distance  .
.
Here,
 ,
,  and
and  are constants. The two terms on the right hand side constitute a repulsion and an attraction, because they are positive and negative, respectively.
are constants. The two terms on the right hand side constitute a repulsion and an attraction, because they are positive and negative, respectively.Richard A. Buckingham proposed this, as a simplification of the Lennard-Jones potential
Lennard-Jones potential
The Lennard-Jones potential is a mathematically simple model that approximates the interaction between a pair of neutral atoms or molecules. A form of the potential was first proposed in 1924 by John Lennard-Jones...
, in a theoretical study of the equation of state for gaseous helium, neon and argon
As explained in Buckingham's original paper and, e.g., in section 2.2.5 of Jensen's text the repulsion is due to the interpenetration of the closed electron shells. "There is therefore some justification for choosing the repulsive part (of the potential) as an exponential function." The Buckingham potential has been used extensively in simulations of molecular dynamics.
Because the exponential term converges to a constant as
 →
→ , while the
, while the 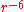 term diverges, the Buckingham potential "turns over" as
term diverges, the Buckingham potential "turns over" as  becomes small. This may be problematic when dealing with a structure with very short interatomic distances, as "nuclear fusion" can occur.
becomes small. This may be problematic when dealing with a structure with very short interatomic distances, as "nuclear fusion" can occur.
