Brooker's merocyanine
Encyclopedia
Brooker's merocyanine is an organic
dye
belonging to the class of merocyanine
s.
MOED is notable for its solvatochromatic
properties, meaning it changes color depending on the solvent
it is dissolved in. Because of this interesting effect and the fact that it is relatively simple to prepare, its synthesis is suitable as an exercise in an undergraduate organic chemistry
laboratory course.
As shown in the structural formula
, MOED can exist in two resonance forms
: A neutral molecule and a zwitterion
. Research indicates that the zwitterion form is most representative when the compound exists in polar
solvents such as water, and the neutral form when it exists in nonpolar solvents such as chloroform
.
 When MOED is dissolved in various liquids, its color will vary, depending on the solvent and its polarity. In general, the more polar the solvent, the shorter the wavelength
When MOED is dissolved in various liquids, its color will vary, depending on the solvent and its polarity. In general, the more polar the solvent, the shorter the wavelength
s of the light absorbed will be. When light of a certain color (wavelength) is absorbed, the solution will appear in the complementary color of the one absorbed. Therefore, in water, a highly polar solvent, MOED appears yellow (corresponding to absorbed blue light of wavelengths 435-480 nm), but is purple or blue (corresponding to absorbed green to yellow light of wavelengths 560-595 nm) in acetone
, a less polar solvent. The effect stems in part from the stabilization of the ground state
of the merocyanine molecule in polar solvents, which increases the energy gap between the ground state and excited state
s, which corresponds to shorter wavelengths (increased energy) of the absorbed light.
sensors and transition metal
cation indicators. Research into merocyanine dyes is ongoing.
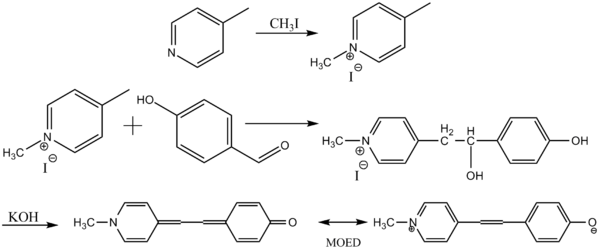
of 4-methylpyridine
to produce 1,4-dimethylpyridium iodide. Reaction with 4-hydroxybenzaldehyde
and subsequent treatment with aqueous base
provides Brooker's merocyanine.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
belonging to the class of merocyanine
Merocyanine
Merocyanines are a class of fluorescent dyes typified by merocycanine I.These dyes are usually intensely colored and have large extinction coefficients....
s.
MOED is notable for its solvatochromatic
Solvatochromism
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity. Negative solvatochromism corresponds to hypsochromic shift with increasing solvent polarity and positive solvatochromism corresponds to bathochromic shift with increasing solvent polarity...
properties, meaning it changes color depending on the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
it is dissolved in. Because of this interesting effect and the fact that it is relatively simple to prepare, its synthesis is suitable as an exercise in an undergraduate organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
laboratory course.
As shown in the structural formula
Structural formula
The structural formula of a chemical compound is a graphical representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly...
, MOED can exist in two resonance forms
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
: A neutral molecule and a zwitterion
Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
. Research indicates that the zwitterion form is most representative when the compound exists in polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
solvents such as water, and the neutral form when it exists in nonpolar solvents such as chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
.
Solvatochromatic effects

Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s of the light absorbed will be. When light of a certain color (wavelength) is absorbed, the solution will appear in the complementary color of the one absorbed. Therefore, in water, a highly polar solvent, MOED appears yellow (corresponding to absorbed blue light of wavelengths 435-480 nm), but is purple or blue (corresponding to absorbed green to yellow light of wavelengths 560-595 nm) in acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, a less polar solvent. The effect stems in part from the stabilization of the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
of the merocyanine molecule in polar solvents, which increases the energy gap between the ground state and excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
s, which corresponds to shorter wavelengths (increased energy) of the absorbed light.
Uses
Because of its solvatochromatic properties MOED, and solvatochromatic dyes in general, are useful as solvent polarity indicators, and for creating solutions that absorb light at a specific frequency. Additional potential areas of use include pHPH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
sensors and transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
cation indicators. Research into merocyanine dyes is ongoing.
Synthesis
Brooker's merocyanine can be prepared beginning with the methylationMethylation
In the chemical sciences, methylation denotes the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group. Methylation is a form of alkylation with, to be specific, a methyl group, rather than a larger carbon chain, replacing a hydrogen atom...
of 4-methylpyridine
4-Methylpyridine
4-Methylpyridine is the organic compound with the formula CH3C5H4N. It is one of the three isomers of methylpyridine. This colourless pungent liquid is a building block for the synthesis of other heterocyclic compounds...
to produce 1,4-dimethylpyridium iodide. Reaction with 4-hydroxybenzaldehyde
4-Hydroxybenzaldehyde
4-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde. It can be found in the orchid Gastrodia elata.-Chemistry:The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a...
and subsequent treatment with aqueous base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
provides Brooker's merocyanine.