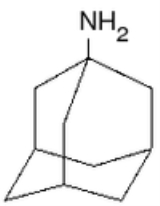
Bridged compounds
Encyclopedia
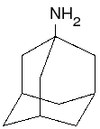

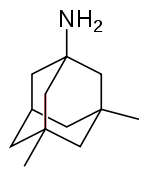
Examples include:
- AdamantaneAdamantaneAdamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
- AmantadineAmantadineAmantadine is the organic compound known formally as 1-adamantylamine or 1-aminoadamantane. The molecule consists of adamantane backbone that has an amino group substituted at one of the four methyne positions. This pharmaceutical is sold under the name Symmetrel for use both as an antiviral and an...
- BiperidenBiperidenBiperiden is an antiparkinsonian agent of the anticholinergic type. The original brand name, which still exists and is manufactured by BASF/Knoll Pharma, is Akineton. Generics are available worldwide.- Pharmacokinetics :...
- Memantine
- Methenamine
- RimantadineRimantadineRimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Both rimantadine and the similar drug...
- NorbornaneNorbornaneNorbornane is an organic compound and a saturated hydrocarbon with chemical formula C7H12. It is a crystalline compound with melting point 88 °C. The carbon skeleton is a cyclohexane ring bridged by a methylene group in the 1,4- position, and is a bridged bicyclic compound...
Nomenclature
The nomenclature of bridged compounds was established by the von Baeyer system. Some important concepts for bridged compounds are:- Bridgehead: Any skeletal atom of the ring system which is bonded to three or more skeletal atoms (excluding hydrogen). In consequence, the bridgeheads are always tertiary or quaternary carbons. For insaturations, Bredt's ruleBredt's RuleBredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough...
states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. - Bridge: Unbranched chain of atoms or an atom or a valence bond connecting two bridgeheads. They may consist in carbon or heteroatoms. (Excluding hydrogen)
- Main ring: Ring identified as the pattern structure.
- Main bridge: Bridge which connects the two bridgeheads in pattern structure.
- Secondary bridge: Any bridge not included in the main ring or the main bridge.
External links
- Rule A-31. Bridged hydrocarbons. Bicyclic Systems http://www.acdlabs.com/iupac/nomenclature/79/r79_163.htm IUPAC Blue Book
- Rule A-32. Bridged Hydrocarbons. Polycyclic Systems http://www.acdlabs.com/iupac/nomenclature/79/r79_164.htm IUPAC Blue Book
- Rule A-34. Hydrocarbon Bridges. Bridged Hydrocarbons http://www.acdlabs.com/iupac/nomenclature/79/r79_166.htm IUPAC Blue Book
- Rule B-14. Bridged Heterocyclic Systems. Extension of the von Baeyer System. http://www.acdlabs.com/iupac/nomenclature/79/r79_994.htm IUPAC Blue Book

