
Blister agent
Encyclopedia
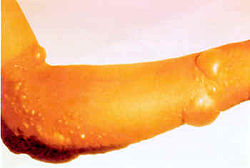
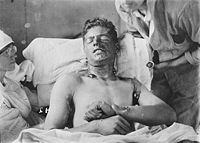
Chemical burn
A chemical burn occurs when living tissue is exposed to a corrosive substance such as a strong acid or base. Chemical burns follow standard burn classification and may cause extensive tissue damage. The main types of irritant and/or corrosive products are: acids, bases, oxidizers, solvents,...
s, resulting in painful water blister
Blister
A blister is a small pocket of fluid within the upper layers of the skin, typically caused by forceful rubbing , burning, freezing, chemical exposure or infection. Most blisters are filled with a clear fluid called serum or plasma...
s on the bodies of those affected. Although the term is often used in connection with large-scale burns caused by chemical spills or chemical warfare agents, some naturally occurring substances such as cantharidin
Cantharidin
Cantharidin, a type of terpenoid, is a poisonous chemical compound secreted by many species of blister beetle, and most notably by the Spanish fly, Lytta vesicatoria. The false blister beetles and cardinal beetles also have cantharidin.-History:...
are also blister-producing agents (vesicants). Vesicants have medical uses including wart removal but can be fatal if even small amounts are ingested.
Blister agents used in warfare
Most blister agents fall into one of three groups:- Sulfur mustards – A family of sulfurSulfurSulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
-based agents, including the so-called "mustard gas". - Nitrogen mustardNitrogen mustardThe nitrogen mustards are cytotoxic chemotherapy agents similar to mustard gas. Although their common use is medicinal, in principle these compounds can also be deployed as chemical warfare agents. Nitrogen mustards are nonspecific DNA alkylating agents. Nitrogen mustard gas was stockpiled by...
s – A family of agents similar to the sulfur mustards, but based on nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
instead of sulfur. - LewisiteLewisiteLewisite is an organoarsenic compound, specifically an arsine. It was once manufactured in the U.S. and Japan as a chemical weapon, acting as a vesicant and lung irritant...
– An early blister agent that was developed, but not used during World War IWorld War IWorld War I , which was predominantly called the World War or the Great War from its occurrence until 1939, and the First World War or World War I thereafter, was a major war centred in Europe that began on 28 July 1914 and lasted until 11 November 1918...
. It was effectively rendered obsolete with the development of British anti-LewisiteDimercaprolDimercaprol or British anti-Lewisite , is a compound developed by British biochemists at Oxford University during World War II. It was developed secretly as an antidote for lewisite, the now-obsolete arsenic-based chemical warfare agent. Today, it is used medically in treatment of arsenic,...
in the 1940s.
Occasionally, phosgene oxime
Phosgene oxime
Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids...
is included among the blister agents, although it is more properly termed a nettle agent
Nettle agent
A nettle agent or urticant is a variety of chemical warfare agent that produces corrosive skin and tissue injury upon contact, resulting in erythema, urticaria, intense itching and a hive-like rash....
.
Effects of blister agents
Exposure to a weaponized blister agent can cause a number of life-threatening symptoms, including:- Severe skin, eye and mucosal pain and irritation
- Skin erythemaErythemaErythema is redness of the skin, caused by hyperemia of the capillaries in the lower layers of the skin. It occurs with any skin injury, infection, or inflammation...
with large fluid blisterBlisterA blister is a small pocket of fluid within the upper layers of the skin, typically caused by forceful rubbing , burning, freezing, chemical exposure or infection. Most blisters are filled with a clear fluid called serum or plasma...
s that heal slowly and may become infected - TearingTearsTears are secretions that clean and lubricate the eyes. Lacrimation or lachrymation is the production or shedding of tears....
, conjunctivitisConjunctivitisConjunctivitis refers to inflammation of the conjunctiva...
, corneaCorneaThe cornea is the transparent front part of the eye that covers the iris, pupil, and anterior chamber. Together with the lens, the cornea refracts light, with the cornea accounting for approximately two-thirds of the eye's total optical power. In humans, the refractive power of the cornea is...
l damage - Mild respiratory distressRespiratory distressRespiratory distress may refer to either/both:* Labored breathing, the physical presentation of respiratory distress*Shortness of breath or dyspnea - a sensation of respiratory distress-See also:*List of terms of lung size and activity...
to marked airway damage
All blister agents currently known are heavier than air, and are readily absorbed through the eyes, lungs, and skin. Effects of the two mustard agents are typically delayed: exposure to vapors becomes evident in 4 to 6 hours, and skin exposure in 2 to 48 hours. The effects of Lewisite are immediate.

