
Biocompatibility
Encyclopedia
Biocompatibility is related to the behavior of biomaterials in various contexts. The term may refer to specific properties of a material without specifying where or how the material is used (for example, that it elicits little or no immune response in a given organism, or is able to integrate with a particular cell type or tissue
), or to more empirical clinical success of a whole device in which the material or materials feature. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body
and eventually how those interactions determine the clinical success of a medical device
(such as pacemaker
, hip replacement
or stent
). Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material.
Indeed, since the immune response and repair functions in the body are so complicated it is not adequate to describe the biocompatibility of a single material in relation to a single cell type or tissue. Sometimes one hears of biocompatibility testing that is a large battery of in vitro
test that is used in accordance with ISO 10993
(or other similar standards) to determine if a certain material (or rather biomedical product) is biocompatible. These tests do not determine the biocompatibility of a material, but they constitute an important step towards the animal testing
and finally clinical trial
s that will determine the biocompatibility of the material in a given application, and thus medical device
s such as implant
s or drug delivery devices.
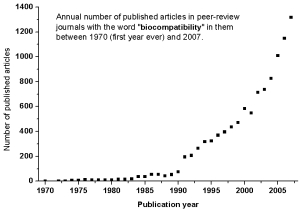
The word biocompatibility seems have been mentioned for the first time in peer-review journals and meetings in 1970 by RJ Hegyeli (Amer Chem Soc Annual Meeting abstract) and CA Homsy et al. ( J Macromol Sci Chem A4:3,615, 1970). It took almost two decades before it began to be commonly used in scientific literature (see the graph below).
Recently Williams (again) has been trying to re-evaluate the current knowledge status regarding what factors determine clinical success. Doing so notes that an implant may not always have to be positively bioactive but it must not do any harm (either locally or systematically) (Williams, 2008).
All these definitions deal with materials and not with devices. This is a drawback since many medical devices are made of more than one material. Much of the pre-clinical testing of the materials is not conducted on the devices but rather the material itself. But at some stage the testing will have to include the device since the shape, geometry and surface treatment etc. of the device will also affect its biocompatibility.
Biocompatibility of long-term implanted devices
Biocompatibility of short-term implantable devices
Biocompatibility of tissue-engineering
products
In these definitions the notion of biocompatibility is related to devices rather than to materials as compared to top three definitions. There was a consensus conference on biomaterial definitions in Sorrento September 15–16, 2005.
Tissue (biology)
Tissue is a cellular organizational level intermediate between cells and a complete organism. A tissue is an ensemble of cells, not necessarily identical, but from the same origin, that together carry out a specific function. These are called tissues because of their identical functioning...
), or to more empirical clinical success of a whole device in which the material or materials feature. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body
Human body
The human body is the entire structure of a human organism, and consists of a head, neck, torso, two arms and two legs.By the time the human reaches adulthood, the body consists of close to 100 trillion cells, the basic unit of life...
and eventually how those interactions determine the clinical success of a medical device
Medical device
A medical device is a product which is used for medical purposes in patients, in diagnosis, therapy or surgery . Whereas medicinal products achieve their principal action by pharmacological, metabolic or immunological means. Medical devices act by other means like physical, mechanical, thermal,...
(such as pacemaker
Pacemaker
An artificial pacemaker is a medical device that uses electrical impulses to regulate the beating of the heart.Pacemaker may also refer to:-Medicine:...
, hip replacement
Hip replacement
Hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant. Hip replacement surgery can be performed as a total replacement or a hemi replacement. Such joint replacement orthopaedic surgery generally is conducted to relieve arthritis pain or fix severe...
or stent
Stent
In the technical vocabulary of medicine, a stent is an artificial 'tube' inserted into a natural passage/conduit in the body to prevent, or counteract, a disease-induced, localized flow constriction. The term may also refer to a tube used to temporarily hold such a natural conduit open to allow...
). Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material.
Indeed, since the immune response and repair functions in the body are so complicated it is not adequate to describe the biocompatibility of a single material in relation to a single cell type or tissue. Sometimes one hears of biocompatibility testing that is a large battery of in vitro
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
test that is used in accordance with ISO 10993
ISO 10993
The ISO 10993 set entails a series of standards for evaluating the biocompatibility of a medical device prior to a clinical study . These documents were preceded by the Tripartite agreement and is a part of the harmonisation of the safe use evaluation of medical devices .-List of the standards in...
(or other similar standards) to determine if a certain material (or rather biomedical product) is biocompatible. These tests do not determine the biocompatibility of a material, but they constitute an important step towards the animal testing
Animal testing
Animal testing, also known as animal experimentation, animal research, and in vivo testing, is the use of non-human animals in experiments. Worldwide it is estimated that the number of vertebrate animals—from zebrafish to non-human primates—ranges from the tens of millions to more than 100 million...
and finally clinical trial
Clinical trial
Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...
s that will determine the biocompatibility of the material in a given application, and thus medical device
Medical device
A medical device is a product which is used for medical purposes in patients, in diagnosis, therapy or surgery . Whereas medicinal products achieve their principal action by pharmacological, metabolic or immunological means. Medical devices act by other means like physical, mechanical, thermal,...
s such as implant
Implant (medicine)
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue...
s or drug delivery devices.

The word biocompatibility seems have been mentioned for the first time in peer-review journals and meetings in 1970 by RJ Hegyeli (Amer Chem Soc Annual Meeting abstract) and CA Homsy et al. ( J Macromol Sci Chem A4:3,615, 1970). It took almost two decades before it began to be commonly used in scientific literature (see the graph below).
Recently Williams (again) has been trying to re-evaluate the current knowledge status regarding what factors determine clinical success. Doing so notes that an implant may not always have to be positively bioactive but it must not do any harm (either locally or systematically) (Williams, 2008).
Five definitions of biocompatibility
- "The ability of a material to perform with an appropriate host response in a specific application", Williams' definition.
- "The quality of not having toxic or injurious effects on biological systems".
- "Comparison of the tissue response produced through the close association of the implanted candidate material to its implant site within the host animal to that tissue response recognised and established as suitable with control materials" - ASTM
- "Refers to the ability of a biomaterial to perform its desired function with respect to a medical therapy, without eliciting any undesirable local or systemic effects in the recipient or beneficiary of that therapy, but generating the most appropriate beneficial cellular or tissue response in that specific situation, and optimising the clinically relevant performance of that therapy".
- "Biocompatibility is the capability of a prosthesis implanted in the body to exist in harmony with tissue without causing deleterious changes".
Comments on the above five definitions
- This is also referred to as the Williams' definition. It was defined in the European Society for BiomaterialsEuropean Society for BiomaterialsThe European Society for Biomaterials is a non-profit organisation that encourages research and spread of information regarding research and uses of biomaterials. Founded in 1976. It has approximately 600 members in 27 countries...
Consensus Conference I and can more easily be found in "The Williams dictionary of Biomaterials". - The Dorland Medical definition not recommended according to Williams Dictionary since it only defines biocompatibility as the absence of host response and does not include any desired or positive interactions between the host tissue and the biomaterials.
- The ASTM is not recommended according to Williams Dictionary since it only refers to local tissue responses, in animal models.
- The fourth is an expansion or rather more precise version of the first definition noting both that low toxicity and the one should be aware of the different demands between various medical applications of the same material.
All these definitions deal with materials and not with devices. This is a drawback since many medical devices are made of more than one material. Much of the pre-clinical testing of the materials is not conducted on the devices but rather the material itself. But at some stage the testing will have to include the device since the shape, geometry and surface treatment etc. of the device will also affect its biocompatibility.
Biocompatible
In the literature, one quite often stumbles upon the adjective form: biocompatible. However, according to Williams' definition, this does not make any sense because biocompatibility is contextual, i.e. much more than just the material itself will determine the clinical outcome of the medical device of which the biomaterial is a part. This also points to one of the weaknesses with the current definition because a medical device usually is made of more than one material.Suggested sub-definitions
The scope of the first definition is so wide that D Williams tried to find suitable subgroups of applications in order to be able to make more narrow definitions. In the MDT article from 2003 the chosen supgroups and their definitions were:Biocompatibility of long-term implanted devices
Implant (medicine)
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue...
- The biocompatibility of a long-term implantable medical device refers to the ability of the device to perform its intended function, with the desired degree of incorporation in the host, without eliciting any undesirable local or systemic effects in that host
Biocompatibility of short-term implantable devices
- The biocompatibility of a medical device that is intentionally placed within the cardiovascular system for transient diagnostic or therapeutic purposes refers to the ability of the device to carry out its intended function within flowing blood, with minimal interaction between device and blood that adversely affects device performance, and without inducing uncontrolled activation of cellular or plasma protein cascades.
Biocompatibility of tissue-engineering
Tissue engineering
Tissue engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physio-chemical factors to improve or replace biological functions...
products
- The biocompatibility of a scaffold or matrix for a tissue-engineering products refers to the ability to perform as a substrate that will support the appropriate cellular activity, including the facilitation of molecular and mechanical signalling systems, in order to optimise tissue regeneration, without eliciting any undesirable effects in those cells, or inducing any undesirable local or systemic responses in the eventual host.
In these definitions the notion of biocompatibility is related to devices rather than to materials as compared to top three definitions. There was a consensus conference on biomaterial definitions in Sorrento September 15–16, 2005.
See also
- Biocompatible materialBiocompatible materialIn surgery, a biocompatible material is a synthetic or natural material used to replace part of a living system or to function in intimate contact with living tissue. Biocompatible materials are intended to interface with biological systems to evaluate, treat, augment or replace any tissue, organ...
- BiomaterialBiomaterialA biomaterial is any matter, surface, or construct that interacts with biological systems. The development of biomaterials, as a science, is about fifty years old. The study of biomaterials is called biomaterials science. It has experienced steady and strong growth over its history, with many...
- Medical deviceMedical deviceA medical device is a product which is used for medical purposes in patients, in diagnosis, therapy or surgery . Whereas medicinal products achieve their principal action by pharmacological, metabolic or immunological means. Medical devices act by other means like physical, mechanical, thermal,...
- ImplantImplant (medicine)An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue...
- Medical grade siliconeMedical grade siliconeMedical grade silicones are silicones tested for biocompatibility and are appropriate to be used for medical applications. In the United States, the Food and Drug Administration regulates materials implanted into the body. Medical grade silicones are generally grouped into three categories: non...

