Antiaromaticity
Encyclopedia
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s are cyclic
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
systems containing alternating single and double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s, where the pi electron energy of antiaromatic compounds is higher than that of its open-chain counterpart. Therefore antiaromatic compounds are unstable and highly reactive; often antiaromatic compounds distort themselves out of planarity to resolve this instability. Antiaromatic compounds usually fail Hückel's rule
Hückel's rule
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931...
of aromaticity.
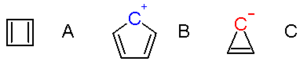
Examples of antiaromatic systems are cyclobutadiene
Cyclobutadiene
Cyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
(A), the cyclopentadienyl cation (B) and the cyclopropenyl anion (C). Cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
is a 4n system but neither aromatic or antiaromatic because the molecule escapes a planar geometry.
By adding or removing an electron pair via a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction, a π system can become aromatic and therefore more stable than the original non- or anti-aromatic compound, for instance the cyclooctatetraenide dianion.
The IUPAC criteria for antiaromaticity are as follows:
- The molecule must have 4n π electrons where n is any integer.
- The molecule must be cyclic.
- The molecule must have a conjugated pi electron system.
- The molecule must be planar.
However, most chemists agree on the definition based on empirical (or simulated) energetic observations.
It is observed that the energy difference between aromatic and antiaromatic compounds diminishes with increasing size. For instance the 12-pi system diphenylene is an antiaromatic compound but stable and even commercially available. The low energy penalty for antiaromaticity is also demonstrated in certain pyrazine
Pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2.Pyrazine is a symmetrical molecule with point group D2h. Derivatives like phenazine are well known for their antitumor, antibiotic and diuretic activity. Pyrazine is less basic in nature than pyridine, pyridazine...
-dihydropyrazine pairs:
The compound on the left is a 14 pi-electron aromatic compound (NICS value –26.1 ppm) which can be reduced in a strongly exothermic reaction
Exothermic reaction
An exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
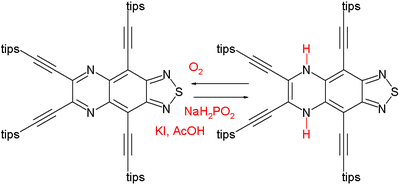
to the 16 pi-electron antiaromatic compound (NICS +27.7 ppm) on the right. The dihydropyrazine slowly converts back to the pyrazine under the action of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. It shows that other electronic factors can overpower aromaticity.
Antiaromaticity is also observed in a chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
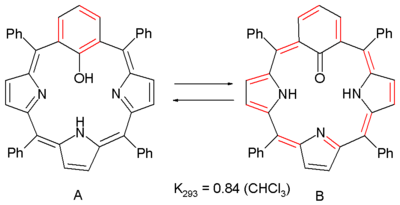
between these two porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
derivatives:
A regular porphyrin is an 18 electron aromatic compound (not counting two non-contributing double bonds) but on substituting a pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
ring by a meta-phenylene ring
Phenylene
The phenylene group is based on a di-substituted benzene ring . For example, poly is a polymer built up from para-phenylene repeating units....
aromaticity is lost due to lack of conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
. In this system the phenylene group is also a phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
and structure A is found to interconvert with 20 electron antiaromat B via keto-enol tautomerism. Antiaromaticity is evident from NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
with the inner NH protons shifting downfield
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
by 10 ppm to 21 ppm. The NICS values compare +0.7 for A (non-aromatic) and +5 (antiaromatic) for B and other computer simulations predict that B is actually more stable than A.