Molisch's test
Encyclopedia
Molisch's Test is a sensitive chemical test
for the presence of carbohydrate
s, based on the dehydration of the carbohydrate by sulfuric acid to produce an aldehyde, which condenses with two molecules of phenol (usually α-naphthol
, though other phenols (e.g. resorcinol
, thymol
) also give colored products) resulting in a red- or purple-colored compound.
dissolved in ethanol) in a test tube
. After mixing, a small amount of concentrated sulfuric acid
is slowly added down the sides of the sloping test-tube, without mixing, to form a bottom layer. A positive reaction is indicated by appearance of a purple ring at the interface between the acid and test layers.
s, disaccharide
s, and polysaccharide
s -- should give a positive reaction, and nucleic acid
s and glycoprotein
s also give a positive reaction, as all these compounds are eventually hydrolyzed to monosacharides by strong mineral acids. Pentose
s are then dehydrated to furfural
, while hexose
s are dehydrated to 5-hydroxymethylfurfural
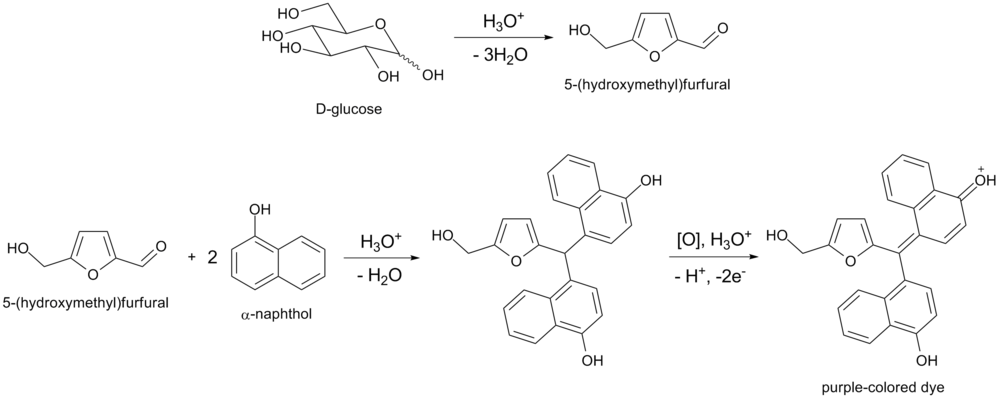
. Either of these aldehydes, if present, will condense with two molecules of naphthol to form a purple-colored product, as illustrated below by the example of glucose
:
Chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to prove the existence of, or to quantify, a chemical compound or chemical group with the aid of a specific reagent...
for the presence of carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s, based on the dehydration of the carbohydrate by sulfuric acid to produce an aldehyde, which condenses with two molecules of phenol (usually α-naphthol
Naphthol
Naphthol may refer to:* 1-Naphthol* 2-Naphthol...
, though other phenols (e.g. resorcinol
Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
, thymol
Thymol
Thymol is a natural monoterpene phenol derivative of cymene, C10H14O, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris and various other kinds of plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties...
) also give colored products) resulting in a red- or purple-colored compound.
Procedure
The test solution is combined with a small amount of Molisch's reagent (α-naphtholNaphthol
Naphthol may refer to:* 1-Naphthol* 2-Naphthol...
dissolved in ethanol) in a test tube
Test tube
A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top, usually with a rounded U-shaped bottom....
. After mixing, a small amount of concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
is slowly added down the sides of the sloping test-tube, without mixing, to form a bottom layer. A positive reaction is indicated by appearance of a purple ring at the interface between the acid and test layers.
Reaction
All carbohydrates -- monosaccharideMonosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s, disaccharide
Disaccharide
A disaccharide or biose is the carbohydrate formed when two monosaccharides undergo a condensation reaction which involves the elimination of a small molecule, such as water, from the functional groups only. Like monosaccharides, disaccharides form an aqueous solution when dissolved in water...
s, and polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s -- should give a positive reaction, and nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s and glycoprotein
Glycoprotein
Glycoproteins are proteins that contain oligosaccharide chains covalently attached to polypeptide side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. In proteins that have segments extending...
s also give a positive reaction, as all these compounds are eventually hydrolyzed to monosacharides by strong mineral acids. Pentose
Pentose
A pentose is a monosaccharide with five carbon atoms. Pentoses are organized into two groups. Aldopentoses have an aldehyde functional group at position 1...
s are then dehydrated to furfural
Furfural
Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source....
, while hexose
Hexose
In organic chemistry, a hexose is a monosaccharide with six carbon atoms, having the chemical formula C6H12O6. Hexoses are classified by functional group, with aldohexoses having an aldehyde at position 1, and ketohexoses having a ketone at position 2....
s are dehydrated to 5-hydroxymethylfurfural
Hydroxymethylfurfural
Hydroxymethylfurfural , also 5-furfural, is an organic compound derived from dehydration of certain sugars. This yellow low-melting solid is highly water-soluble. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups...
. Either of these aldehydes, if present, will condense with two molecules of naphthol to form a purple-colored product, as illustrated below by the example of glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
: