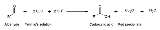
Fehling's solution
Overview
Chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to prove the existence of, or to quantify, a chemical compound or chemical group with the aid of a specific reagent...
used to differentiate between water-soluble aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s, and as a test for monosaccharides. The test was developed by German chemist Hermann von Fehling
Hermann von Fehling
Hermann von Fehling was a German chemist, famous as the developer of Fehling's solution used for estimation of sugar.-Biography:...
in 1849.
Fehling's solution is always prepared fresh in the laboratory. It is made initially as two separate solutions, known as Fehling's A and Fehling's B. Fehling's A is a blue aqueous solution of copper(II) sulfate, while Fehling's B is a clear solution of aqueous potassium sodium tartrate
Potassium sodium tartrate
Potassium sodium tartrate is a double salt first prepared by an apothecary, Pierre Seignette, of La Rochelle, France. As a result the salt was known as Seignette's salt or Rochelle salt....
(also known as Rochelle salt) and a strong alkali (commonly sodium hydroxide).
Equal volumes of the two mixtures are mixed together to get the final Fehling's solution, which is a deep blue colour.

